Demineralized bone matrix combined with concentrated growth factors promotes intervertebral fusion in a novel rat extreme lateral interbody fusion model
- PMID: 40426199
- PMCID: PMC12117761
- DOI: 10.1186/s13018-025-05954-2
Demineralized bone matrix combined with concentrated growth factors promotes intervertebral fusion in a novel rat extreme lateral interbody fusion model
Abstract
Background: Whether demineralized bone matrix (DBM) combined with concentrated growth factors (CGF) can accelerate intervertebral fusion remains uncertain. This study developed a novel rat model for extreme lateral interbody fusion (XLIF) and evaluated the fusion outcomes of DBM combined with CGF using imaging and histological analysis.
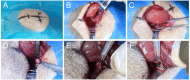
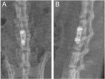
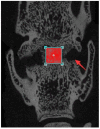
Methods: A total of 70 male SD rats (3 months old, average body weight 300 ± 50 g) were included in this study. Among them, 10 rats were used for the anatomical study of the lumbar spine. The remaining 48 rats were randomly divided into four groups (n = 12 per group): Group A (control), Group B (titanium plate fixation), Group C (DBM + titanium plate fixation), and Group D (DBM + CGF + titanium plate fixation). The remaining 12 rats were used as donors to prepare fresh CGF. Eight weeks after surgery, the rats were euthanized and lumbar spine specimens were collected, with interbody fusion evaluated by manual palpation. Subsequently, specimens from groups B, C, and D were analyzed by micro-CT and histological examinations to comprehensively assess the fusion outcome.
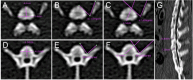
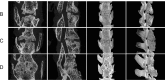
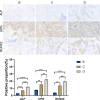
Results: The anatomical and surgical techniques for the rat XLIF model are described. Titanium plates (7 mm × 2.5 mm × 0.8 mm) and screws (3 mm × 1 mm) were designed based on the anatomical measurements. In Group A, spontaneous fusion occurred in 1 case; the remaining 11 cases showed intervertebral mobility. In Group B, 3 cases achieved fusion; in Group C, 8 cases; and in Group D, 11 cases. Micro-CT revealed fusion index scores (FIS) of 2.21 ± 0.51 for Group B, 3.62 ± 0.67 for Group C, and 4.57 ± 0.56 for Group D. Histological examination showed limited bone formation in Group B, with fibrous connective tissue filling the intervertebral space. Group C showed more bone formation, but some cartilage and fibrous tissue remained. Group D demonstrated abundant new bone formation and robust histological fusion, with substantial bridging between vertebrae.
Conclusion: The rat XLIF model for interbody fusion has been successfully established and validated. Using this model, it was preliminarily demonstrated that DBM combined with CGF can effectively promote intervertebral fusion in rats.
Keywords: Concentrated growth factor; Demineralized bone matrix; Intervertebral fusion; XLIF.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All studies have been performed in accordance with the ethical standards in the 1964 Declaration of Helsinki and the relevant regulations of the US Health Insurance Portability and Accountability Act (HIPAA). This experiment obtained ethical approval from the Ethics Committee of the Third Hospital of Hebei Medical University (No. Z2023-026-1). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Improved intervertebral fusion in LLIF rabbit model with a novel titanium cage.Spine J. 2024 Jun;24(6):1109-1120. doi: 10.1016/j.spinee.2023.12.011. Epub 2024 Jan 10. Spine J. 2024. PMID: 38211901
-
Efficacy comparison of Accell Evo3 and Grafton demineralized bone matrix putties against autologous bone in a rat posterolateral spine fusion model.Spine J. 2017 Jun;17(6):855-862. doi: 10.1016/j.spinee.2017.01.012. Epub 2017 Jan 23. Spine J. 2017. PMID: 28126356
-
The osteoinductive properties of Nell-1 in a rat spinal fusion model.Spine J. 2007 Jan-Feb;7(1):50-60. doi: 10.1016/j.spinee.2006.04.020. Epub 2006 Nov 17. Spine J. 2007. PMID: 17197333
-
Evaluation of a new formulation of demineralized bone matrix putty in a rabbit posterolateral spinal fusion model.Spine J. 2014 Sep 1;14(9):2155-63. doi: 10.1016/j.spinee.2014.01.053. Epub 2014 Feb 8. Spine J. 2014. PMID: 24512696
-
Examination of the Role of Cells in Commercially Available Cellular Allografts in Spine Fusion: An in Vivo Animal Study.J Bone Joint Surg Am. 2020 Dec 16;102(24):e135. doi: 10.2106/JBJS.20.00330. J Bone Joint Surg Am. 2020. PMID: 33079897
References
-
- Schnake KJ, Rappert D, Storzer B, Schreyer S, Hilber F, Mehren C. Lumbale Spondylodese– Indikationen und techniken. Orthopäde. 2018;48(1):50–8. - PubMed
-
- Berjano P, Gautschi OP, Schils F, Tessitore E. Extreme lateral interbody fusion (XLIF®): how I do it. Acta Neurochir. 2014;157(3):547–51. - PubMed
-
- Li JXJ, Phan K, Mobbs R. Oblique lumbar interbody fusion: technical aspects, operative outcomes, and complications. World Neurosurg. 2017;98:113–23. - PubMed
-
- Liu X, Zhou J, Chen M, Chen S, You J, Li Y, Lv H, Zhang Y, Zhou Y. 3D-printed biomimetic bone scaffold loaded with lyophilized concentrated growth factors promotes bone defect repair by regulation the VEGFR2/PI3K/AKT signaling pathway. Int J Biol Macromol. 2024;282(Pt 2):136938. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

