AbOmpA in Acinetobacter baumannii: exploring virulence mechanisms of outer membrane-integrated and outer membrane vesicle-associated AbOmpA and developing anti-infective agents targeting AbOmpA
- PMID: 40426208
- PMCID: PMC12108004
- DOI: 10.1186/s12929-025-01147-5
AbOmpA in Acinetobacter baumannii: exploring virulence mechanisms of outer membrane-integrated and outer membrane vesicle-associated AbOmpA and developing anti-infective agents targeting AbOmpA
Abstract
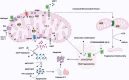
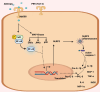
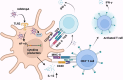
Acinetobacter baumannii is notorious for its antimicrobial resistance and its potential to cause epidemics in hospital settings, which pose a global health threat. Although this microorganism is traditionally considered a low-virulence pathogen, extensive research has been conducted on its virulence and pathogenesis in recent years. Advances in understanding the virulence mechanisms of A. baumannii have prompted a shift in the development of anti-infective agents. The outer membrane protein A (AbOmpA) of A. baumannii is a key virulence factor both in vitro and in vivo. AbOmpA exists in three forms: outer membrane-integrated AbOmpA, outer membrane vesicle (OMV)-associated AbOmpA, and free proteins. Given that outer membrane-integrated AbOmpA has been implicated in the virulence and antimicrobial resistance of A. baumannii, many studies have focused on outer membrane-integrated AbOmpA as a therapeutic target for combating drug-resistant A. baumannii, and have led to the discovery of small molecules, polypeptides, and antimicrobial peptides targeting AbOmpA. However, the pathophysiological role of OMV-associated AbOmpA and its impact on AbOmpA-targeting agents remain unclear. This review summarizes the current knowledge of AbOmpA and critically discusses OMV-associated AbOmpA in relation to virulence and its potential impact on AbOmpA-targeted therapies to provide a better understanding of AbOmpA for the development of novel therapeutics against A. baumannii.
Keywords: Acinetobacter baumannii; Anti-infective agent; Outer membrane protein A; Outer membrane vesicle; Virulence factor.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures

References
-
- Chopra T, Marchaim D, Awali RA, Krishna A, Johnson P, Tansek R, Chaudary K, Lephart P, Slim J, Hothi J, Ahmed H, Pogue JM, Zhao JJ, Kaye KS. Epidemiology of bloodstream infections caused by Acinetobacter baumannii and impact of drug resistance to both carbapenems and ampicillin-sulbactam on clinical outcomes. Antimicrob Agents Chemother. 2013;57:6270–5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

