The Role of Flexibility in the Bioactivity of Short α-Helical Antimicrobial Peptides
- PMID: 40426489
- PMCID: PMC12108317
- DOI: 10.3390/antibiotics14050422
The Role of Flexibility in the Bioactivity of Short α-Helical Antimicrobial Peptides
Abstract
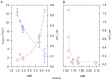
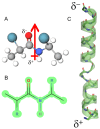
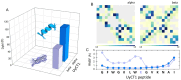
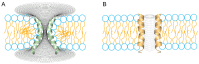
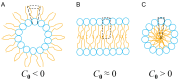
The formation of aqueous pores through the interaction of amphipathic peptides is a process facilitated by the conformational dynamics typical of these biomolecules. Prior to their insertion with the membrane, these peptides go through several conformational states until they finally reach a stable α-helical structure. The conformational dynamics of these pore-forming peptides, α-PFP, is, thus, encoded in their amino acid sequence, which also predetermines their intrinsic flexibility. However, although the role of flexibility is widely recognized as fundamental in their bioactivity, it is still unclear whether this parameter is indeed decisive, as there are reports favoring the view of highly disruptive flexible peptides and others where relative rigidity also predetermines high rates of permeability across membranes. In this review we discuss in depth all those aspects linked to the conformational dynamics of these small biomolecules and which depend on the composition, sequence and dynamic performance both in aqueous phase and in close interaction with phospholipids. In addition, evidence is provided for the contribution of the known carboxyamidation in some well-studied α-PFPs, which are preferentially associated with sequences intrinsically more rigid than those not amidated and generally more flexible than the former. Taken together, this information is of great relevance for the optimization of new antibiotic peptides.
Keywords: dipolar moment; intrinsic flexibility; short antimicrobial peptides.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

