In Vivo Antimicrobial Activity of Nisin Z Against S. aureus and Polyurea Pharmadendrimer PUREG4OEI48 Against P. aeruginosa from Diabetic Foot Infections
- PMID: 40426511
- PMCID: PMC12108245
- DOI: 10.3390/antibiotics14050444
In Vivo Antimicrobial Activity of Nisin Z Against S. aureus and Polyurea Pharmadendrimer PUREG4OEI48 Against P. aeruginosa from Diabetic Foot Infections
Abstract
Background/objectives: Diabetic foot infections (DFIs) are commonly associated with frequent hospitalizations, limb amputations, and premature death due to the profile of the bacteria infecting foot ulcers. DFIs are generally colonized by a polymicrobial net of bacteria that grows in biofilms, developing an increased antimicrobial resistance to multiple antibiotics. DFI treatment is a hurdle, and the need to develop new therapies that do not promote resistance is urgent. Therefore, the antibacterial efficacy of Nisin Z (antimicrobial peptide), a core-shell polycationic polyurea pharmadendrimer (PUREG4OEI48) (antimicrobial polymer), and amlodipine (antihypertensive drug) was evaluated against S. aureus and P. aeruginosa isolated from a DFI and previously characterized.
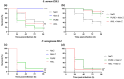
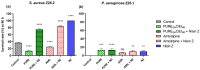
Methods: The antibacterial activity was analyzed in vitro by determining the minimal inhibitory concentration (MIC) and in vivo in a Galleria mellonella model by assessing the larvae survival and health index.
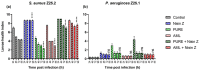
Results: The results indicate that Nisin Z exhibited antibacterial activity against S. aureus in vivo, allowing larvae full survival, and no antibacterial activity against P. aeruginosa. Nisin Z may have reduced the antibacterial effectiveness of both PUREG4OEI48 and amlodipine. PUREG4OEI48 significantly increased the survival of the larvae infected with P. aeruginosa, while amlodipine showed no activity against both bacteria in vivo.
Conclusions: These findings suggest that both Nisin Z and PUREG4OEI48 could potentially be used individually as adjunct treatments for mild DFIs. However, further studies are needed to confirm these findings and assess the potential toxicity and efficacy of PUREG4OEI48 in more complex models.
Keywords: Amlodipine; Galleria mellonella; Nisin Z; Pseudomonas aeruginosa; Staphylococcus aureus; core–shell polycationic polyurea pharmadendrimer; diabetic foot infection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Bacteriostatic and Antibiofilm Efficacy of a Nisin Z Solution against Co-Cultures of Staphylococcus aureus and Pseudomonas aeruginosa from Diabetic Foot Infections.Life (Basel). 2023 Feb 11;13(2):504. doi: 10.3390/life13020504. Life (Basel). 2023. PMID: 36836861 Free PMC article.
-
Diabetic foot infections: Application of a nisin-biogel to complement the activity of conventional antibiotics and antiseptics against Staphylococcus aureus biofilms.PLoS One. 2019 Jul 24;14(7):e0220000. doi: 10.1371/journal.pone.0220000. eCollection 2019. PLoS One. 2019. PMID: 31339915 Free PMC article.
-
Nisin Z Potential for the Control of Diabetic Foot Infections Promoted by Pseudomonas aeruginosa Persisters.Antibiotics (Basel). 2023 Apr 22;12(5):794. doi: 10.3390/antibiotics12050794. Antibiotics (Basel). 2023. PMID: 37237697 Free PMC article.
-
Influence of Storage on the Antimicrobial and Cytotoxic Activities of a Nisin-biogel with Potential to be Applied to Diabetic Foot Infections Treatment.Antibiotics (Basel). 2020 Nov 6;9(11):781. doi: 10.3390/antibiotics9110781. Antibiotics (Basel). 2020. PMID: 33172027 Free PMC article.
-
The effect of nisin on the biofilm production, antimicrobial susceptibility and biofilm formation of Staphylococcus aureus and Pseudomonas aeruginosa.Eur J Med Res. 2022 Sep 8;27(1):173. doi: 10.1186/s40001-022-00804-x. Eur J Med Res. 2022. PMID: 36076252 Free PMC article.
References
-
- Magliano D.J., Boyko E.J., IDF Diabetes Atlas 10th Edition Scientific Committee . IDF Diabetes Atlas. 10th ed. International Diabetes Federation; Brussels, Belgium: 2021.
Grants and funding
- UIDB/00276/2020/FCT/CIISA-Centro de Investigação Interdisciplinar em Sanidade Animal
- LA/P/0059/2020 - AL4AnimalS/FCT/Laboratório Associado para Ciência Animal e Veterinária
- PTDC/SAUINF/28466/2017; PTDC/MEC-ONC/29327/2017/Fundação para a Ciência e a Tecnologia (FCT)
- UIDB/04565/2020; UIDP/04565/2020/FCT/Research Unit Institute for Bioengineering and Biosciences (iBB)
- LA/P/0140/2020/FCT/Associate Laboratory Institute for Health and Bioeconomy (i4HB)
LinkOut - more resources
Full Text Sources

