Heterogeneity of Biofilm Formation Among Staphylococcus aureus and Coagulase-Negative Staphylococcus Species in Clinically Relevant Intravenous Fat Emulsions
- PMID: 40426550
- PMCID: PMC12108170
- DOI: 10.3390/antibiotics14050484
Heterogeneity of Biofilm Formation Among Staphylococcus aureus and Coagulase-Negative Staphylococcus Species in Clinically Relevant Intravenous Fat Emulsions
Abstract
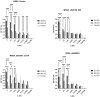
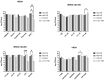
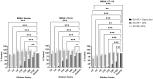
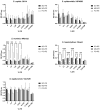
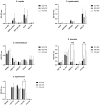
Background: Compared to soybean oil intravenous fat emulsion (SO-IFE), use of mixed-oil IFE (MO-IFE) is associated with reduced rates of catheter-related bloodstream infections caused by coagulase-negative Staphylococcus species (CoNS) in pediatric patients receiving parenteral nutrition. Methods: Using an in vitro biofilm model, this study aimed to assess the impact of IFEs on biofilm formation among Staphylococcus species. S. aureus, S. capitis, S. epidermidis, S. haemolyticus, S. hominis, and S. lugdunensis were cultivated as biofilms in media supplemented with SO-IFE, MO-IFE, or fish oil IFE (IFE). Biomass was quantified by the crystal violet method, and follow-up planktonic growth assays assessed antimicrobial effects of IFEs. Results: Compared to SO-IFE, MO-IFE and FO-IFE significantly inhibited biofilm formation of S. aureus but did not impact planktonic growth. Contrary to clinical data, CoNS biofilm formation was not impacted by any of the IFEs tested. S. aureus biofilm inhibition in IFEs was further investigated by comparing differences following growth in SO-IFE supplemented with capric acid, docosahexaenoic acid (DHA), or eicosapenaenoic acid (EPA) to concentrations matching those of MO-IFE. Capric acid supplementation was associated with significant reduction in biofilm formation compared to SO-IFE alone. However, this was attributed to a bactericidal effect based on follow-up planktonic growth assays. Conclusions: These results suggest that biofilm formation in S. aureus is variably impacted by fatty acid composition in clinically relevant IFEs, with capric acid exhibiting bactericidal activity against tested isolates.
Keywords: Staphylococcus; biofilm; catheter-related infections; fat emulsions; intravenous; parenteral nutrition.
Conflict of interest statement
J.S.S. reports grant funding from Merck Sharp and Dohme for research outside of this work. The other authors declare no conflicts of interest.
Figures


References
-
- Mehta N.M., Skillman H.E., Irving S.Y., Coss-Bu J.A., Vermilyea S., Farrington E.A., McKeever L., Hall A.M., Goday P.S., Braunschweig C. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Pediatric Critically Ill Patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. JPEN J. Parenter. Enteral Nutr. 2017;41:706–742. doi: 10.1177/0148607117711387. - DOI - PubMed
-
- Advani S., Reich N.G., Sengupta A., Gosey L., Milstone A.M. Central Line-Associated Bloodstream Infection in Hospitalized Children with Peripherally Inserted Central Venous Catheters: Extending Risk Analyses Outside the Intensive Care Unit. Clin. Infect. Dis. 2011;52:1108–1115. doi: 10.1093/cid/cir145. - DOI - PMC - PubMed
-
- Netto R., Mondini M., Pezzella C., Romani L., Lucignano B., Pansani L., D’Argenio P., Cogo P. Parenteral nutrition is one of the most significant risk factors for nosocomial infections in a pediatric cardiac intensive care unit. JPEN J. Parenter. Enteral Nutr. 2017;41:612–618. doi: 10.1177/0148607115619416. - DOI - PubMed
Grants and funding
- R21AI153768/National Institutes of Allergy and Infectious Disease
- UTHSC collaborative seed grant/University of Tennessee Health Science Center College of Pharmacy
- Foundation Futures grant/American College of Clinical Pharmacy
- NA/University of Tennessee Health Science Center's Center for Pediatric Experimental Therapeutics
- R01AI177615/National Institutes of Allergy and Infectious Disease
LinkOut - more resources
Full Text Sources
Research Materials

