In Vitro Activity of Imipenem/Relebactam Alone and in Combination Against Cystic Fibrosis Isolates of Mycobacterium abscessus
- PMID: 40426552
- PMCID: PMC12108374
- DOI: 10.3390/antibiotics14050486
In Vitro Activity of Imipenem/Relebactam Alone and in Combination Against Cystic Fibrosis Isolates of Mycobacterium abscessus
Abstract
Background: Mycobacterium abscessus (MABS) is an opportunistic pathogen that causes chronic, difficult-to-treat pulmonary infections, particularly in people with cystic fibrosis (PwCF), leading to rapid lung function decline and increased morbidity and mortality. Treatment is particularly challenging due to the pathogen's resistance mechanisms and the need for prolonged multidrug therapy, which is characterized by poor clinical outcomes and highlights the urgent need for novel therapeutic strategies. Imipenem/relebactam, a novel β-lactam-β-lactamase inhibitor combination, demonstrates in vitro activity against resistant MABS strains and effective pulmonary penetration. Prior research indicates synergistic activity of imipenem with various antibiotics against M. abscessus.
Objectives: This study aims to evaluate the in vitro activity of imipenem/relebactam, alone and in combination with various antibiotics, against MABS clinical isolates from PwCF (n = 28).
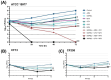
Methods: Susceptibility and synergy were assessed using broth microdilution and checkerboard assays. Extracellular time-kill assays were performed to evaluate the bactericidal activity of synergistic three-drug combinations containing imipenem/relebactam.
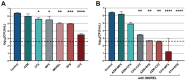
Results: Imipenem/relebactam demonstrated potent in vitro activity against clinical MABS isolates, exhibiting substantial synergy with cefuroxime, cefdinir, amoxicillin, and cefoxitin. Rifabutin, azithromycin, moxifloxacin, clofazimine, and minocycline also demonstrated additive effects with imipenem/relebactam. Extracellular time-kill assays identified imipenem/relebactam + cefoxitin + rifabutin and imipenem/relebactam + cefoxitin + moxifloxacin as the most effective combinations.
Conclusions: These findings suggest that imipenem/relebactam may offer a significant advancement in the management of MABS infections in PwCF. The promising efficacy of multidrug regimens combining imipenem/relebactam with agents like cefoxitin, azithromycin, moxifloxacin, clofazimine, and rifabutin highlights potential therapeutic strategies.
Keywords: Mycobacterium abscessus; combination therapy; cystic fibrosis; imipenem/relebactam; synergy.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
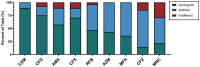
References
-
- Floto R.A., Olivier K.N., Saiman L., Daley C.L., Herrmann J.L., Nick J.A., Noone P.G., Bilton D., Corris P., Gibson R.L., et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax. 2016;71((Suppl. S1)):i1–i22. doi: 10.1136/thoraxjnl-2015-207360. - DOI - PMC - PubMed
-
- Cystic Fibrosis Foundation 2023 Patient Registry: Annual Data Report. 2024. [(accessed on 9 May 2025)]. Available online: https://www.cff.org/media/34491/download.
Grants and funding
LinkOut - more resources
Full Text Sources

