Efficacy and Safety of Prolonged Adjuvant Temozolomide Treatment in Glioblastoma: Prospective Study of 81 Patients Undergoing up to 101 Cycles of Treatment
- PMID: 40426599
- PMCID: PMC12110070
- DOI: 10.3390/brainsci15050428
Efficacy and Safety of Prolonged Adjuvant Temozolomide Treatment in Glioblastoma: Prospective Study of 81 Patients Undergoing up to 101 Cycles of Treatment
Abstract
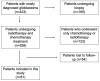
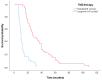
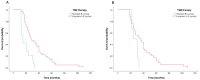
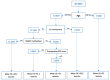
Background: Although several studies investigated the efficacy of long-term adjuvant temozolomide (TMZ) therapy in glioblastomas (GBs), no univocal data are currently available, and this topic remains controversial. The present study on our ongoing experience aims to assess whether the extended STUPP protocol confers prognostic benefits with acceptable safety. Methods: From 2004 to 2018, 81 patients with a new diagnosis of GB according to the World Health Organization (WHO) 2021 classification, treated with gross total resection (GTR) or subtotal resection (STR), were enrolled. Patients were divided into Group A (long-term TMZ; N = 40) and Group B (standard STUPP protocol; N = 41). Results: In the extended STUPP group, compared with the standard STUPP group, progression-free survival (PFS) and overall survival (OS) were significantly improved (PFS: 27.8 vs. 7.5 months, p = 0.00001; OS: 35.9 vs. 11.3 months, p = 0.0001). To mitigate a potential survival bias, we focused on those in Group B who completed the recommended six cycles. Patients in Group A demonstrated a prolonged OS compared to Group B (27 vs. 10 months, p < 0.001). Similar findings were observed in a focused analysis of patients who had achieved a minimum survival of 12 months (27 vs. 15 months, p < 0.001) or 18 months (34 vs. 24 months, p = 0.044). Conclusions: Our analysis demonstrates a PFS and OS advantage with extended STUPP and suggests that young patients without corpus callosum invasion, with methylguanine-DNA methyltransferase (MGMT) promoter methylation, and treated with GTR are the best candidates. No significant safety difference emerged between extended and standard TMZ treatment.
Keywords: STUPP; extended; glioblastoma; long-term; temozolomide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Gately L., Mesía C., Sepúlveda J.M., Del Barco S., Pineda E., Gironés R., Fuster J., Hong W., Dumas M., Gill S., et al. A combined analysis of two prospective randomised studies exploring the impact of extended post-radiation temozolomide on survival outcomes in newly diagnosed glioblastoma. J. Neurooncol. 2024;166:407–415. doi: 10.1007/s11060-023-04513-1. Erratum in J. Neurooncol. 2024, 166, 417–418. - DOI - PubMed
-
- Karschnia P., Young J.S., Dono A., Häni L., Sciortino T., Bruno F., Juenger S.T., Teske N., Morshed R.A., Haddad A.F., et al. Prognostic validation of a new classification system for extent of resection in glioblastoma: A report of the RANO resect group. Neuro-Oncology. 2023;25:940–954. doi: 10.1093/neuonc/noac193. - DOI - PMC - PubMed
-
- Basch E., Becker C., Rogak L.J., Schrag D., Reeve B.B., Spears P., Smith M.L., Gounder M.M., Mahoney M.R., Schwartz G.K., et al. Composite grading algorithm for the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) Clin. Trials. 2021;18:104–114. doi: 10.1177/1740774520975120. - DOI - PMC - PubMed
-
- Wen P.Y., Macdonald D.R., Reardon D.A., Cloughesy T.F., Sorensen A.G., Galanis E., DeGroot J., Wick W., Gilbert M.R., Lassman A.B., et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

