Chronic Stress Modulates Microglial Activation Dynamics, Shaping Priming Responses to Subsequent Stress
- PMID: 40426704
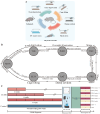
- PMCID: PMC12110633
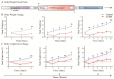
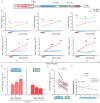
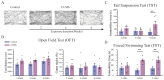
- DOI: 10.3390/brainsci15050534
Chronic Stress Modulates Microglial Activation Dynamics, Shaping Priming Responses to Subsequent Stress
Abstract
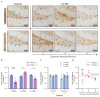
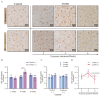
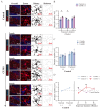
(1) Background: The high recurrence rate and individual differences in stress susceptibility contribute to the diverse symptoms of depression, making full recovery and relapse prevention challenging. Emerging evidence suggests that fluctuations in microglial activity are closely linked to depression progression under chronic stress exposure. Changes in the brain microenvironment can elicit microglial priming, enhancing their sensitivity to external stimuli. However, few studies have longitudinally examined how microglial characteristics evolve throughout depression progression. (2) Methods: In this study, we investigated microglial morphological changes and their responses to acute stress at different stages of depression using the chronic unpredictable mild stress (CUMS) paradigm in mice. (3) Results: Our findings reveal that in the dentate gyrus, microglial activation indices, including cell number and morphology, exhibit distinct dynamic patterns depending on CUMS exposure duration. Notably, after 2 and 4 weeks of CUMS exposure followed by acute stress re-exposure, microglia display opposing response patterns. In contrast, after 6 weeks of CUMS exposure, primed microglia exhibit dysfunction, failing to respond to acute stress. Notably, depressive behaviors are not prominent after 2 weeks of CUMS exposure but become more pronounced after 4 and 6 weeks of exposure. Additionally, regardless of CUMS duration, body weight demonstrates an intrinsic capacity to normalize after stress cessation. (4) Conclusions: These findings suggest that microglial priming responses are state-dependent, either enhancing or suppressing secondary stimulus responses, or exceeding physiological limits, thereby preventing further activation. This study provides novel insights into the role of microglial priming in stress vulnerability and its contribution to depression progression.
Keywords: chronic unpredictable mild stress (CUMS); depression; microglia; microglial priming; stress vulnerability.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Priming of microglia with dysfunctional gut microbiota impairs hippocampal neurogenesis and fosters stress vulnerability of mice.Brain Behav Immun. 2024 Jan;115:280-294. doi: 10.1016/j.bbi.2023.10.031. Epub 2023 Oct 31. Brain Behav Immun. 2024. PMID: 37914097
-
Microglial-glucocorticoid receptor depletion alters the response of hippocampal microglia and neurons in a chronic unpredictable mild stress paradigm in female mice.Brain Behav Immun. 2021 Oct;97:423-439. doi: 10.1016/j.bbi.2021.07.022. Epub 2021 Jul 31. Brain Behav Immun. 2021. PMID: 34343616
-
SRT2104 attenuates chronic unpredictable mild stress-induced depressive-like behaviors and imbalance between microglial M1 and M2 phenotypes in the mice.Behav Brain Res. 2020 Jan 27;378:112296. doi: 10.1016/j.bbr.2019.112296. Epub 2019 Oct 13. Behav Brain Res. 2020. PMID: 31618623
-
Knockdown of FSTL1 inhibits microglia activation and alleviates depressive-like symptoms through modulating TLR4/MyD88/NF-κB pathway in CUMS mice.Exp Neurol. 2022 Jul;353:114060. doi: 10.1016/j.expneurol.2022.114060. Epub 2022 Mar 31. Exp Neurol. 2022. PMID: 35367454
-
Minocycline ameliorates depressive behaviors and neuro-immune dysfunction induced by chronic unpredictable mild stress in the rat.Behav Brain Res. 2019 Jan 1;356:348-357. doi: 10.1016/j.bbr.2018.07.001. Epub 2018 Jul 9. Behav Brain Res. 2019. PMID: 30003978
References
LinkOut - more resources
Full Text Sources

