Probiotic, Prebiotic, and Synbiotic Supplementation for the Prevention and Treatment of Acute Otitis Media: A Systematic Review and Meta-Analysis
- PMID: 40426770
- PMCID: PMC12109743
- DOI: 10.3390/children12050591
Probiotic, Prebiotic, and Synbiotic Supplementation for the Prevention and Treatment of Acute Otitis Media: A Systematic Review and Meta-Analysis
Abstract
Background and aim: Probiotics, prebiotics, and synbiotics have been documented to modulate the microbiota, enhance immunity, and reduce antibiotic resistance, making them a promising alternative in the management of acute otitis media (AOM). Accordingly, the aim of this study was to determine their effectiveness in the prevention and treatment of AOM in patients.
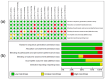
Methods: A systematic review and meta-analysis of randomized controlled trials published between 2000 and 2024 was conducted using Science Direct, PubMed, LILACS, SCOPUS, Web of Science, and Cochrane Clinical Trials, following PRISMA guidelines. The methodological quality was evaluated using the Jadad scale, and the meta-analysis was performed with RevMan 5.4® and Jamovi 2.3.28®.
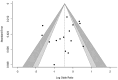
Results: A total of 16 trials with 4034 patients were included. The meta-analysis showed that the intervention did not affect the time to AOM presentation (MD: -7.98; 95% CI: -19.74 to 3.78; p = 0.18), the recurrence of the disease (RR: 0.99; 95% CI: 0.74-1.33; p = 0.96), or the requirement for antibiotics (RR: 1.31; 95% CI: 0.92 to 1.84; p = 0.13); however, it was associated with a reduced probability of developing AOM (RR: 0.80; 95% CI: 0.66 to 0.96; p = 0.02). Subgroup analysis suggests that the effect of probiotic supplementation on AOM incidence is influenced by treatment duration, patient age, and the number of probiotic strains in the product.
Conclusions: Supplementation with probiotics, prebiotics, or synbiotics is associated with a significant reduction in the incidence of AOM in children, although no significant impact was observed on other key clinical parameters. These interventions may be considered as a complementary strategy to conventional treatments; however, further high-quality, standardized trials are needed to confirm these findings and to define optimal protocols.
Keywords: acute otitis media; dysbiosis; microbiota; prebiotics; probiotics; systematic review.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Folino F., Di Pasquale D., Marchisio P., Pignataro L., Capaccio P., Gaini L., Battilocchi L., Bosis S., Torretta S. Topical administration of S. salivarius 24SMB—S. oralis 89a in children with adenoidal disease: A double-blind controlled trial. Eur. J. Pediatr. 2024;183:289–294. doi: 10.1007/s00431-023-05192-w. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

