The Risk of Vestibular Disorders with Semaglutide and Tirzepatide: Findings from a Large Real-World Cohort
- PMID: 40426877
- PMCID: PMC12109458
- DOI: 10.3390/biomedicines13051049
The Risk of Vestibular Disorders with Semaglutide and Tirzepatide: Findings from a Large Real-World Cohort
Abstract
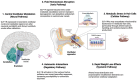
Background/Objectives: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have revolutionized the treatment of type 2 diabetes and obesity. While their metabolic benefits are well-established, their potential effects on vestibular function remain unexplored. This study investigated the association between GLP-1RA use and the risk of vestibular disorders. Methods: Using the TriNetX research network (accessed 3 November 2024), we conducted a retrospective cohort study of adults prescribed semaglutide (n = 419,497) or tirzepatide (n = 77,259) between January 2018 and October 2024. Cases were matched 1:1 with controls using propensity scores based on demographics and comorbidities. The primary outcome was new-onset vestibular disorders, analyzed at 6 months, 1 year, and 3 years after treatment initiation. Results: Both medications were associated with an increased risk of vestibular disorders. Semaglutide users showed a higher cumulative incidence (0.12% at 6 months to 0.41% at 3 years) compared to controls (0.03% to 0.16%, p < 0.001), with hazard ratios ranging from 4.02 (95% CI: 3.33-4.86) at 6 months to 4.95 (95% CI: 4.51-5.43) at 3 years. Tirzepatide users demonstrated similar patterns but lower absolute rates (0.10% at 6 months to 0.19% at 3 years vs. controls 0.04% to 0.15%), with hazard ratios from 3.19 (95% CI: 2.11-4.81) to 4.55 (95% CI: 3.43-6.03). The direct comparison showed a higher risk with semaglutide versus tirzepatide (RR 1.53-2.04, p < 0.001). Conclusions: GLP-1RA therapy is associated with an increased risk of vestibular disorders, with a higher risk observed with semaglutide compared to tirzepatide. These findings suggest the need for vestibular symptom monitoring in patients receiving these medications and warrant further investigation into underlying mechanisms.
Keywords: GLP-1 receptor agonists; adverse effects; dizziness; obesity; pharmacovigilance; semaglutide; tirzepatide; type 2 diabetes; vertigo; vestibular disorders.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Wen S., Nguyen T., Gong M., Yuan X., Wang C., Jin J., Zhou L. An Overview of Similarities and Differences in Metabolic Actions and Effects of Central Nervous System Between Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RAs) and Sodium Glucose Co-Transporter-2 Inhibitors (SGLT-2is) Diabetes Metab. Syndr. Obes. Targets Ther. 2021;14:2955–2972. doi: 10.2147/DMSO.S312527. - DOI - PMC - PubMed
-
- Olukorode J.O., Orimoloye D.A., Nwachukwu N.O., Onwuzo C.N., Oloyede P.O., Fayemi T., Odunaike O.S., Ayobami-Ojo P.S., Divine N., Alo D.J., et al. Recent Advances and Therapeutic Benefits of Glucagon-Like Peptide-1 (GLP-1) Agonists in the Management of Type 2 Diabetes and Associated Metabolic Disorders. Cureus. 2024;16:e72080. doi: 10.7759/cureus.72080. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

