The Effects of the Biological Agents Infliximab, Vedolizumab, and Ustekinumab on Intestinal Anastomosis: An Experimental Study in Rats
- PMID: 40426907
- PMCID: PMC12109263
- DOI: 10.3390/biomedicines13051079
The Effects of the Biological Agents Infliximab, Vedolizumab, and Ustekinumab on Intestinal Anastomosis: An Experimental Study in Rats
Abstract
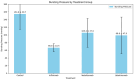
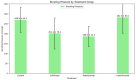
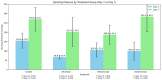
Background/Objectives: The potential side effects of the use of biological agents in the perioperative period are still under investigation. This animal prospective study aimed to evaluate the overall impact of biological factor administration after intestinal surgery. Methods: This study included 80 female Wistar rats sorted into four groups: three groups received one of the biological factors, infliximab, vedolizumab, or ustekinumab; the control group received placebo therapy. After enterotomy and intestinal anastomosis, the bursting pressure (BP) of the anastomosis was compared among the groups on postoperative days (PODs) 3 and 7. Results: On POD3, the control group presented with a significantly higher mean BP (154.6 ± 39.7 mmHg) compared to the infliximab (66.8 ± 10.4 mmHg), vedolizumab (105.4 ± 37.6 mmHg), and ustekinumab (98.8 ± 47.9 mmHg) groups. A post hoc analysis among the three biological agent groups revealed differences only when comparing infliximab and vedolizumab rats with the controls on POD3 (p < 0.001) and with the ustekinumab rats on POD7, having a greater mean BP (282.5 ± 80.1 mmHg, p = 0.031). No differences were observed regarding the event of broken anastomosis among the four groups. Conclusions: This experimental study's findings highlight the varying detrimental effects of different biological agents on the strength of intestinal anastomosis, with ustekinumab demonstrating superior performance.
Keywords: IBDs; biological agents; experimental study; infliximab; surgical complications; ustekinumab; vedolizumab.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Superior treatment persistence with ustekinumab in Crohn's disease and vedolizumab in ulcerative colitis compared with anti-TNF biological agents: real-world registry data from the Persistence Australian National IBD Cohort (PANIC) study.Aliment Pharmacol Ther. 2021 Aug;54(3):292-301. doi: 10.1111/apt.16436. Epub 2021 Jun 20. Aliment Pharmacol Ther. 2021. PMID: 34151447
-
Combination Therapy With Immunomodulators Improves the Pharmacokinetics of Infliximab But Not Vedolizumab or Ustekinumab.Clin Gastroenterol Hepatol. 2023 Oct;21(11):2908-2917.e10. doi: 10.1016/j.cgh.2022.10.016. Epub 2022 Oct 22. Clin Gastroenterol Hepatol. 2023. PMID: 36280102
-
Anti-TNF Agents and New Biological Agents (Vedolizumab and Ustekinumab) in the Prevention and Treatment of Postoperative Recurrence After Surgery in Crohn's Disease.Drugs. 2023 Sep;83(13):1179-1205. doi: 10.1007/s40265-023-01916-2. Epub 2023 Jul 28. Drugs. 2023. PMID: 37505446 Free PMC article. Review.
-
Dose escalation of biologics in biologic-naive patients with Crohn's disease: Outcomes from the ODESSA-CD study.J Manag Care Spec Pharm. 2024 Nov;30(11):1276-1287. doi: 10.18553/jmcp.2024.30.11.1276. J Manag Care Spec Pharm. 2024. PMID: 39471266 Free PMC article.
-
Selection strategy of second-line biologic therapies in adult patients with ulcerative colitis following prior biologic treatment failure: Systematic review and meta-analysis.Pharmacol Res. 2024 Apr;202:107108. doi: 10.1016/j.phrs.2024.107108. Epub 2024 Feb 24. Pharmacol Res. 2024. PMID: 38403257
References
LinkOut - more resources
Full Text Sources