Tumor-Associated Macrophages as Key Modulators of Disease Progression in Diffuse Large B-Cell Lymphoma
- PMID: 40426926
- PMCID: PMC12108958
- DOI: 10.3390/biomedicines13051099
Tumor-Associated Macrophages as Key Modulators of Disease Progression in Diffuse Large B-Cell Lymphoma
Abstract
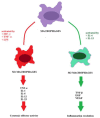
With the advent of new therapeutic approaches, there is hope that anticancer treatment will eventually be possible without the use of chemotherapy. Efficient immunotherapeutic options have recently emerged in many cancers, offering a less aggressive approach, with overall better tolerance, making them also suitable for frail patients. Response to immunotherapy relies on the availability, functionality, and efficacy of the host's immune effector mechanisms. One of the key factors determining the efficacy of immunotherapy is the tumor microenvironment, which encompasses various immune effectors, including macrophages, which play a crucial role in regulating immune responses through phagocytosis and antigen presentation. Macrophages are prototypically divided, according to their polarization, into either the pro-inflammatory M1 type or the anti-inflammatory M2 type. In the tumor microenvironment, M2-polarized macrophages, known as tumor-associated macrophages (TAMs), are the predominant phenotype and are associated with tumor progression. The M1/M2 paradigm contributes to the understanding of tumor progression. Due to the variable microenvironment, the mechanisms regulating TAMs can vary across different cancers. Variations in TAM polarization may account for the different treatment responses in patients with similar diseases. This paper investigates the connection between TAMs, disease progression, and treatment responses in the most frequent solid hematologic cancer, diffuse large B-cell lymphoma.
Keywords: TAMs; macrophage polarization; non-Hodgkin lymphoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Targeting tumor-associated macrophages for cancer immunotherapy.Int Rev Cell Mol Biol. 2022;368:61-108. doi: 10.1016/bs.ircmb.2022.02.002. Epub 2022 Apr 27. Int Rev Cell Mol Biol. 2022. PMID: 35636930
-
Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes.Theranostics. 2021 Jan 1;11(6):2892-2916. doi: 10.7150/thno.50928. eCollection 2021. Theranostics. 2021. PMID: 33456579 Free PMC article.
-
Autophagy drives plasticity and functional polarization of tumor-associated macrophages.IUBMB Life. 2022 Feb;74(2):157-169. doi: 10.1002/iub.2543. Epub 2021 Aug 31. IUBMB Life. 2022. PMID: 34467634 Review.
-
Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy.Front Immunol. 2022 Jun 30;13:888713. doi: 10.3389/fimmu.2022.888713. eCollection 2022. Front Immunol. 2022. PMID: 35844605 Free PMC article. Review.
-
Antitumor Research Based on Drug Delivery Carriers: Reversing the Polarization of Tumor-Associated Macrophages.Mol Pharm. 2025 Mar 3;22(3):1174-1197. doi: 10.1021/acs.molpharmaceut.4c01277. Epub 2025 Jan 27. Mol Pharm. 2025. PMID: 39868820 Review.
References
Publication types
LinkOut - more resources
Full Text Sources

