Patient-Oriented In Vitro Studies in Duchenne Muscular Dystrophy: Validation of a 3D Skeletal Muscle Organoid Platform
- PMID: 40426938
- PMCID: PMC12109395
- DOI: 10.3390/biomedicines13051109
Patient-Oriented In Vitro Studies in Duchenne Muscular Dystrophy: Validation of a 3D Skeletal Muscle Organoid Platform
Abstract
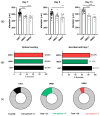
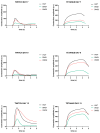
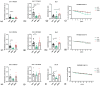
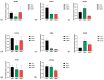
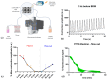
Background: Three-dimensional skeletal muscle organoids (3D SkMO) are becoming of increasing interest for preclinical studies in Duchenne muscular dystrophy (DMD), provided that the used platform demonstrates the possibility to form functional and reproducible 3D SkMOs, to investigate on potential patient-related phenotypic differences. Methods: In this study, we employed fibrin-based 3D skeletal muscle organoids derived from immortalized myogenic precursors of DMD patients carrying either a stop codon mutation in exon 59 or a 48-50 deletion. We compared dystrophic lines with a healthy wild-type control (HWT) by assessing microtissue formation ability, contractile function at multiple timepoints along with intracellular calcium dynamics via calcium imaging, as well as expression of myogenic markers. Results: We found patient-specific structural and functional differences in the early stages of 3D SkMO development. Contractile force, measured as both single twitch and tetanic responses, was significantly lower in dystrophic 3D SkMOs compared to HWT, with the most pronounced differences observed at day 7 of differentiation. However, these disparities diminished over time under similar culturing conditions and in the absence of continuous nerve-like stimulation, suggesting that the primary deficit lies in delayed myogenic maturation, as also supported by gene expression analysis. Conclusions: Our results underline that, despite the initial maturation delay, DMD muscle precursors retain the capacity to form functional 3D SkMOs once this intrinsic lag is overcome. This suggests a critical role of dystrophin in early myogenic development, while contraction-induced stress and/or an inflammatory microenvironment are essential to fully recapitulate dystrophic phenotypes in 3D SkMOs.
Keywords: 3D skeletal muscle organoid; Duchenne muscular dystrophy; disease modeling; immortalized human myoblast; tissue engineering.
Conflict of interest statement
Authors from BIOND Solutions B.V. had a supporting role in the study design and manuscript revision. All other authors declare no competing interests.
Figures


Similar articles
-
De novo revertant fiber formation and therapy testing in a 3D culture model of Duchenne muscular dystrophy skeletal muscle.Acta Biomater. 2021 Sep 15;132:227-244. doi: 10.1016/j.actbio.2021.05.020. Epub 2021 May 25. Acta Biomater. 2021. PMID: 34048976
-
Mimicking sarcolemmal damagein vitro: a contractile 3D model of skeletal muscle for drug testing in Duchenne muscular dystrophy.Biofabrication. 2023 Sep 27;15(4). doi: 10.1088/1758-5090/acfb3d. Biofabrication. 2023. PMID: 37725998
-
Engineered skeletal muscle recapitulates human muscle development, regeneration and dystrophy.J Cachexia Sarcopenia Muscle. 2022 Dec;13(6):3106-3121. doi: 10.1002/jcsm.13094. Epub 2022 Oct 18. J Cachexia Sarcopenia Muscle. 2022. PMID: 36254806 Free PMC article.
-
The X-linked Becker muscular dystrophy (bmx) mouse models Becker muscular dystrophy via deletion of murine dystrophin exons 45-47.J Cachexia Sarcopenia Muscle. 2023 Apr;14(2):940-954. doi: 10.1002/jcsm.13171. Epub 2023 Jan 11. J Cachexia Sarcopenia Muscle. 2023. PMID: 36628607 Free PMC article.
-
Molecular pathways involved in the control of contractile and metabolic properties of skeletal muscle fibers as potential therapeutic targets for Duchenne muscular dystrophy.Front Physiol. 2024 Dec 9;15:1496870. doi: 10.3389/fphys.2024.1496870. eCollection 2024. Front Physiol. 2024. PMID: 39717824 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

