Regulation of Kv2.1 Channels by Kv9.1 Variants
- PMID: 40426946
- PMCID: PMC12108608
- DOI: 10.3390/biomedicines13051119
Regulation of Kv2.1 Channels by Kv9.1 Variants
Abstract
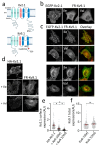
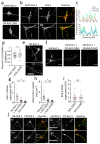
Background/Objectives: Kv2 channels have important conducting and nonconducting functions and are regulated by their co-assembly with 'silent' Kv subunits, including Kv9.1. Kv9.1 is co-expressed with Kv2 channels in sensory neurons, and a common allele that changes Ile489 to Val in human Kv9.1 is associated with pain hypersensitivity in patients. The mechanism responsible for this association remains unknown, but we hypothesise that these two variants differ in their regulation of Kv2.1 properties, and this is what we set out to test. Methods: Expression was carried out using HEK293 cells, OHeLa cells, and primary cultures of hippocampal neurons, and the biophysical and trafficking properties of homomeric and heteromeric channels were assessed by confocal fluorescence microscopy and patch clamp analysis. Results: Both Kv9.1Ile and Kv9.1Val were retained within the endoplasmic reticulum when expressed individually, but when co-expressed with Kv2.1, they co-localised with Kv2.1 within the surface clusters. Both variants reduced the surface expression of Kv2.1 channels and the size of channel clusters, with Kv9.1Val producing a greater reduction in surface expression in both the HeLa cells and neurons. They both caused a similar hyperpolarising shift in the voltage dependence of channel activation and inactivation. Concatamers of Kv2.1 and Kv9.1 suggested that both 3:1 and 2:2 ratios of Kv2.1 to Kv9.1 were permitted, although 2:2 resulted in lower surface expression and function. Conclusions: The Ile489Val substitution in Kv9.1 does not disrupt its ability to co-assemble with Kv2 channels, nor its effects on the voltage-dependence of channel gating, but it did produce a greater reduction in the Kv2.1 surface expression, suggesting that this underlies its association with pain hypersensitivity.
Keywords: Kv2 potassium channels; fluorescence imaging; neurons; pain; patch clamp electrophysiology.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Heteromeric assembly of Kv2.1 with Kv9.3: effect on the state dependence of inactivation.Biophys J. 1999 Jul;77(1):248-57. doi: 10.1016/S0006-3495(99)76886-4. Biophys J. 1999. PMID: 10388754 Free PMC article.
-
Modification of delayed rectifier potassium currents by the Kv9.1 potassium channel subunit.Hear Res. 2000 Sep;147(1-2):21-30. doi: 10.1016/s0378-5955(00)00117-9. Hear Res. 2000. PMID: 10962170
-
Structural determinants of the regulation of the voltage-gated potassium channel Kv2.1 by the modulatory α-subunit Kv9.3.J Biol Chem. 2003 May 16;278(20):18154-61. doi: 10.1074/jbc.M213117200. Epub 2003 Mar 17. J Biol Chem. 2003. PMID: 12642579
-
Kv5, Kv6, Kv8, and Kv9 subunits: No simple silent bystanders.J Gen Physiol. 2016 Feb;147(2):105-25. doi: 10.1085/jgp.201511507. Epub 2016 Jan 11. J Gen Physiol. 2016. PMID: 26755771 Free PMC article. Review.
-
Adam, amigo, brain, and K channel.Biophys Rev. 2023 Nov 6;15(5):1393-1424. doi: 10.1007/s12551-023-01163-5. eCollection 2023 Oct. Biophys Rev. 2023. PMID: 37975011 Free PMC article. Review.
References
-
- Bocksteins E., Raes A.L., Van de Vijver G., Bruyns T., Van Bogaert P.-P., Snyders D.J. Kv2. 1 and silent Kv subunits underlie the delayed rectifier K+ current in cultured small mouse DRG neurons. Am. J. Physiol.-Cell Physiol. 2009;296:C1271–C1278. doi: 10.1152/ajpcell.00088.2009. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

