Cortical Thickness Changes in Migraine Patients Treated with Anti-Calcitonin Gene-Related Peptide Monoclonal Antibodies: A Prospective Age- and Sex-Matched Controlled Study
- PMID: 40426977
- PMCID: PMC12108776
- DOI: 10.3390/biomedicines13051150
Cortical Thickness Changes in Migraine Patients Treated with Anti-Calcitonin Gene-Related Peptide Monoclonal Antibodies: A Prospective Age- and Sex-Matched Controlled Study
Abstract
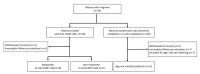
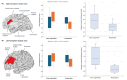
Background: Migraine is associated with structural brain abnormalities, including cortical thickness changes. Anti-calcitonin gene-related peptide monoclonal antibodies (anti-CGRP mAbs) are a novel therapy for migraine prevention, but their effects on cortical structures are poorly understood. Methods: In this prospective age- and sex-matched controlled study, 30 migraine patients receiving either anti-CGRP mAbs (fremanezumab) (n = 15) or oral preventive medications (n = 15) underwent 3T MRI scans before and after treatment. Treatment response was defined as a ≥50% reduction in monthly headache days after 3 months. Cortical thickness was analyzed across 46 cortical regions, comparing patients treated with anti-CGRP mAbs to those receiving oral preventive treatment, as well as responders to non-responders within the anti-CGRP group. Results: Cortical thickness changes did not differ significantly between the anti-CGRP and oral treatment groups. However, among patients receiving anti-CGRP mAbs, responders showed significant decreases in cortical thickness compared to non-responders, particularly in the right caudal anterior cingulate (p = 0.026) and left rostral middle frontal cortex (p = 0.007). These cortical changes correlated with treatment response to anti-CGRP mAbs (β = -0.429, 95% CI [-0.777, -0.081], p = 0.016 in the right caudal anterior cingulate; β = -0.224, 95% CI [-0.390, -0.057], p = 0.008 in the left rostral middle frontal cortex). Conclusions: This exploratory study, based on a small sample size, suggests that cortical thickness changes may be associated with treatment response to anti-CGRP mAbs rather than with CGRP mAb treatment itself. Further studies with larger cohorts are needed to confirm these findings.
Keywords: anti-calcitonin gene-related peptide monoclonal antibody; cortical thickness; migraine; prospective study; treatment.
Conflict of interest statement
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Peripherally acting anti-CGRP monoclonal antibodies alter cortical gray matter thickness in migraine patients: A prospective cohort study.Neuroimage Clin. 2023;40:103531. doi: 10.1016/j.nicl.2023.103531. Epub 2023 Oct 14. Neuroimage Clin. 2023. PMID: 37866119 Free PMC article.
-
Real-world persistence and costs among patients with chronic migraine treated with onabotulinumtoxinA or calcitonin gene-related peptide monoclonal antibodies.J Manag Care Spec Pharm. 2023 Oct;29(10):1119-1128. doi: 10.18553/jmcp.2023.29.10.1119. J Manag Care Spec Pharm. 2023. PMID: 37776119 Free PMC article.
-
Safety of Rimegepant, an Oral CGRP Receptor Antagonist, Plus CGRP Monoclonal Antibodies for Migraine.Headache. 2020 Sep;60(8):1734-1742. doi: 10.1111/head.13930. Epub 2020 Aug 16. Headache. 2020. PMID: 32799325 Free PMC article. Clinical Trial.
-
Monoclonal antibodies blocking CGRP transmission: An update on their added value in migraine prevention.Rev Neurol (Paris). 2020 Dec;176(10):788-803. doi: 10.1016/j.neurol.2020.04.027. Epub 2020 Aug 2. Rev Neurol (Paris). 2020. PMID: 32758365 Review.
-
Calcitonin gene-related peptide monoclonal antibody for preventive treatment of episodic migraine: A meta analysis.Clin Neurol Neurosurg. 2017 Mar;154:74-78. doi: 10.1016/j.clineuro.2017.01.009. Epub 2017 Jan 22. Clin Neurol Neurosurg. 2017. PMID: 28129635 Review.
References
-
- Stovner L.J., Nichols E., Steiner T.J., Abd-Allah F., Abdelalim A., Al-Raddadi R.M., Ansha M.G., Barac A., Bensenor I.M., Doan L.P. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954–976. doi: 10.1016/S1474-4422(18)30322-3. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

