Biomarker-Driven Approaches to Bone Metastases: From Molecular Mechanisms to Clinical Applications
- PMID: 40426987
- PMCID: PMC12109438
- DOI: 10.3390/biomedicines13051160
Biomarker-Driven Approaches to Bone Metastases: From Molecular Mechanisms to Clinical Applications
Abstract
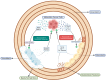
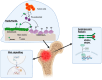
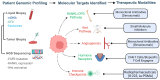
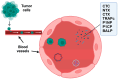
Bone metastases represent a critical complication in oncology, frequently indicating advanced malignancy and substantially reducing patient quality of life. This review provides a comprehensive analysis of the complex interactions between tumor cells and the bone microenvironment, emphasizing the relevance of the "seed and soil" hypothesis, the RANK/RANKL/OPG signaling axis, and Wnt signaling pathways that collectively drive metastatic progression. The molecular and cellular mechanisms underlying the formation of osteolytic and osteoblastic lesions are examined in detail, with a particular focus on their implications for bone metastases associated with breast, prostate, lung, and other cancers. A central component of this review is the categorization of pathological biomarkers into four types: diagnostic, prognostic, predictive, and monitoring. We provide a comprehensive evaluation of circulating tumor cells (CTCs), bone turnover markers (such as TRACP-5b and CTX), advanced imaging biomarkers (including PET/CT and MRI), and novel genomic signatures. These biomarkers offer valuable insights for early detection, enhanced risk stratification, and optimized therapeutic decision-making. Furthermore, emerging strategies in immunotherapy and bone-targeted treatments are discussed, highlighting the potential of biomarker-guided precision medicine to enhance personalized patient care. The distinctiveness of this review lies in its integrative approach, combining fundamental pathophysiological insights with the latest developments in biomarker discovery and therapeutic innovation. By synthesizing evidence across various cancer types and biomarker categories, we provide a cohesive framework aimed at advancing both the scientific understanding and clinical management of bone metastases.
Keywords: BTMs; CTCs; biomarkers in precision oncology; bone metastasis; circulating biomarkers; ctDNA; precision medicine; therapeutic targets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
Advancements in Biomarkers for Early Detection and Risk Stratification of Cardiovascular Diseases-A Literature Review.Health Sci Rep. 2025 May 26;8(5):e70878. doi: 10.1002/hsr2.70878. eCollection 2025 May. Health Sci Rep. 2025. PMID: 40432692 Free PMC article.
-
Biochemical Markers of Osteoporosis.2024 Nov 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Nov 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 32644732 Free Books & Documents.
-
Dual Biomarker Strategies for Liquid Biopsy: Integrating Circulating Tumor Cells and Circulating Tumor DNA for Enhanced Tumor Monitoring.Biosensors (Basel). 2025 Jan 28;15(2):74. doi: 10.3390/bios15020074. Biosensors (Basel). 2025. PMID: 39996976 Free PMC article. Review.
-
Balancing the Scales: The Dual Role of Interleukins in Bone Metastatic Microenvironments.Int J Mol Sci. 2024 Jul 26;25(15):8163. doi: 10.3390/ijms25158163. Int J Mol Sci. 2024. PMID: 39125732 Free PMC article. Review.
References
-
- Brown J.E., Cook R.J., Major P., Lipton A., Saad F., Smith M., Lee K.-A., Zheng M., Hei Y.-J., Coleman R.E. Bone Turnover Markers as Predictors of Skeletal Complications in Prostate Cancer, Lung Cancer, and Other Solid Tumors. J. Natl. Cancer Inst. 2005;97:59–69. doi: 10.1093/jnci/dji002. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

