Laser-Induced Cortical Lesions in Mice as a Model for Progressive Multiple Sclerosis Pathology
- PMID: 40427022
- PMCID: PMC12109324
- DOI: 10.3390/biomedicines13051195
Laser-Induced Cortical Lesions in Mice as a Model for Progressive Multiple Sclerosis Pathology
Abstract
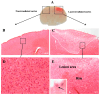
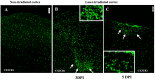
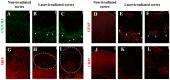
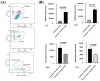
Background: The current animal models of multiple sclerosis (MS) predominantly emphasize white matter inflammation, reflecting early-stage disease. However, progressive MS (PMS) is characterized by cortical pathology, including subpial demyelination, chronic meningeal inflammation, and microglial activation, which are underrepresented in the existing models. While alternative mouse models replicate the relapsing-remitting phenotype and gray matter pathology, pathology is frequently dispersed throughout the brain, complicating the analysis of the specific lesion sites. Methods: To address this gap, we developed a novel model that integrates laser-induced focal demyelination with cytokine-driven meningeal inflammation to replicate the key aspects of PMS cortical pathology. Results: Using two-photon laser irradiation, we induced controlled subpial cortical lesions in CX3CR1-GFP mice, leading to microglial activation, astrocytosis, and focal demyelination. The addition of IFNγ-expressing adenovirus to promote meningeal inflammation which resulted in prolonged glial responses, increased immune cell infiltration, and exacerbated demyelination, mimicking the PMS-associated pathology. Conclusions: This model provides a powerful tool to investigate the mechanisms underlying the cortical lesion development and immune-mediated neurodegeneration in PMS. By capturing the critical aspects of cortical pathology, it enables the evaluation of therapeutic strategies targeting neuroinflammation and demyelination, ultimately aiding in the development of new treatments of progression in PMS patients.
Keywords: cortical pathology; demyelination; meningeal inflammation; progressive multiple sclerosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

