Effectiveness of Systemic Corticosteroids in Managing Cancer-Related Neuropathic Pain: A Multicenter Prospective Observational Study
- PMID: 40427128
- PMCID: PMC12109982
- DOI: 10.3390/cancers17101630
Effectiveness of Systemic Corticosteroids in Managing Cancer-Related Neuropathic Pain: A Multicenter Prospective Observational Study
Abstract
Background: Cancer-related neuropathic pain (CR-NP) is challenging to manage, and the effectiveness of corticosteroids remains underexplored.
Objectives: This study investigated the analgesic and functional benefits of corticosteroids in CR-NP.
Methods: This multicenter, prospective observational study enrolled patients with CR-NP who initiated or escalated corticosteroid therapy. Pain intensity and daily activities were assessed at baseline (T0), 72 (T1), and 168 h (T2). A paired-sample t-test compared pain intensity changes. Linear regression analysis examined the association between changes in opioid daily dose and pain intensity, while Pearson's correlation coefficient assessed the relationship between changes in daily activities and pain intensity.
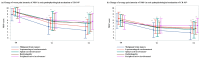
Results: In total, 107 patients were consecutively enrolled. The mean worst pain intensity decreased from 8.2 ± 1.9 at T0 to 5.2 ± 2.9 at T1 and further to 4.4 ± 3.0 at T2. No significant correlation was found between changes in opioid daily dose and pain intensity. However, daily activities improved significantly in correlation with pain reduction (r = -0.36, p < 0.01). Over 75% of patients reported satisfaction with CR-NP management. Adverse events occurred in 21 cases and were generally mild.
Conclusions: Corticosteroids provided rapid and considerable analgesic and functional benefits for patients with CR-NP in this observational setting; further validation through comparative controlled studies is required.
Keywords: brain; cancer; cancer pain; corticosteroids; inflammation; neuropathic pain; neuropathy; palliative care; primary sensory neurons; spinal cord.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

References
-
- World Health Organization . Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. World Health Organization; Geneva, Switzerland: 2018. - PubMed
-
- Tagami K., Chiu S.W., Kosugi K., Ishiki H., Hiratsuka Y., Shimizu M., Mori M., Kubo E., Ikari T., Arakawa S., et al. Cancer pain management in patients receiving inpatient specialized palliative care services. J. Pain Symptom Manag. 2024;67:27–38.e1. doi: 10.1016/j.jpainsymman.2023.09.015. - DOI - PubMed
-
- Roberto A., Deandrea S., Greco M.T., Corli O., Negri E., Pizzuto M., Ruggeri F. Prevalence of neuropathic pain in cancer patients: Pooled estimates from a systematic review of published literature and results from a survey conducted in 50 Italian palliative care centers. J. Pain Symptom Manag. 2016;51:1091–1102.e4. doi: 10.1016/j.jpainsymman.2015.12.336. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

