Tea Polyphenols Mitigate Radiation-Induced Ferroptosis and Intestinal Injury by Targeting the Nrf2/HO-1/GPX4 Signaling Pathway
- PMID: 40427462
- PMCID: PMC12108355
- DOI: 10.3390/antiox14050580
Tea Polyphenols Mitigate Radiation-Induced Ferroptosis and Intestinal Injury by Targeting the Nrf2/HO-1/GPX4 Signaling Pathway
Abstract
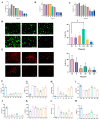
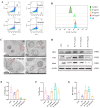
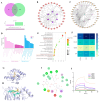
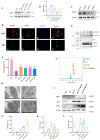
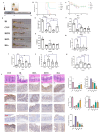
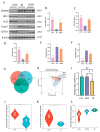
Radiation-induced intestinal injury (RIII) is a significant concern for cancer patients receiving radiation therapy, as it can lead to complications such as radiation enteropathy. Presently, there are limited options for preventing or treating RIII. Tea polyphenols (TP), found in tea, provide various health benefits, but their antiradiation mechanisms are not fully understood. C57BL/6 mice pre-treated with TP for five days showed a significant improvement in survival rates after being exposed to 10 Gy of 60Co radiation. In the same way, abdominal exposure to 15 Gy of 60Co radiation effectively mitigated radiation-induced colon shortening, damage to intestinal tissues, oxidative stress, the release of inflammatory factors, and disruptions in intestinal microbial balance. In addition, TP treatment lowered the elevation of reactive oxygen species (ROS), iron imbalance, mitochondrial damage, and ferroptosis in IEC-6 cells post-irradiation. Utilizing network pharmacology, molecular docking, and affinity testing, we identified that TP has the capability to target the Nrf2/HO-1/GPX4 signaling pathway, while EGCG, a principal constituent of TP, interacts with HSP90 and mitigates radiation-induced ferroptosis. These findings suggest that TP may serve as a promising therapeutic agent to alleviate radiation-induced intestinal injury (RII).
Keywords: HSP90; ferroptosis; gut microbiota; metabolites; radiation-induced intestinal injury; tea polyphenol.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Green tea derivative (-)-epigallocatechin-3-gallate (EGCG) confers protection against ionizing radiation-induced intestinal epithelial cell death both in vitro and in vivo.Free Radic Biol Med. 2020 Dec;161:175-186. doi: 10.1016/j.freeradbiomed.2020.10.012. Epub 2020 Oct 15. Free Radic Biol Med. 2020. PMID: 33069855
-
Total extracts from Abelmoschus manihot (L.) alleviate radiation-induced cardiomyocyte ferroptosis via regulating redox imbalances mediated by the NOX4/xCT/GPX4 axis.J Ethnopharmacol. 2024 Nov 15;334:118582. doi: 10.1016/j.jep.2024.118582. Epub 2024 Jul 14. J Ethnopharmacol. 2024. PMID: 39009325
-
Protective effect of total flavonoids of Engelhardia roxburghiana Wall. leaves against radiation-induced intestinal injury in mice and its mechanism.J Ethnopharmacol. 2023 Jul 15;311:116428. doi: 10.1016/j.jep.2023.116428. Epub 2023 Mar 28. J Ethnopharmacol. 2023. PMID: 36997130
-
Interactions of tea polyphenols with intestinal microbiota and their implication for cellular signal conditioning mechanism.J Food Biochem. 2019 Aug;43(8):e12953. doi: 10.1111/jfbc.12953. Epub 2019 Jun 17. J Food Biochem. 2019. PMID: 31368563 Review.
-
Interactions of tea polyphenols with intestinal microbiota and their effects on cerebral nerves.J Food Biochem. 2021 Jan;45(1):e13575. doi: 10.1111/jfbc.13575. Epub 2020 Nov 21. J Food Biochem. 2021. PMID: 33222220 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

