Manganese Porphyrin Treatment Improves Redox Status Caused by Acute Compressive Spinal Cord Trauma
- PMID: 40427469
- PMCID: PMC12108513
- DOI: 10.3390/antiox14050587
Manganese Porphyrin Treatment Improves Redox Status Caused by Acute Compressive Spinal Cord Trauma
Abstract
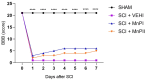
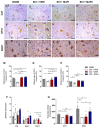
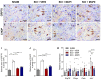
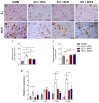
There is increasing interest in identifying drugs that can prevent or delay neurological complications following spinal cord injury, thus expanding the therapeutic window for other potential neuroprotective agents. In this context, manganese porphyrins (MnPs) have shown high antioxidant and anti-inflammatory potential in various experimental disease models, including stroke, cancer, diabetes, ischemia, and radiotherapy. However, they have been little evaluated in spinal cord injuries. This study aimed to assess the therapeutic potential of the manganese porphyrins [MnTE-2-PyP]5+ (MnPI) and [MnT(5-Br-3-E-Py)P]5+ (MnPII) in acute compressive spinal cord trauma in rats. Twenty-four animals were used (six animals/group). Following general inhalation anesthesia, acute compressive spinal cord trauma was induced in all groups except for the negative control (SHAM). Treatment commenced 60 min post-trauma, with animals receiving treatment for seven days at 24 h intervals. While no improvement in motor capacity was observed, MnPs effectively blocked the increase in oxidative stress and endoplasmic reticulum (ER) stress mediators caused by trauma, maintaining the protein expression levels of Hifα, 8-OHdG and MDA, as well as the expression of the genes Grp78, Chop, Ho1, and Perk, similar to those of the control group. Moreover, there was an increase in protein expression of SOD1, Cat, and GPX1, along with a restoration of SOD and CAT enzymatic activity. Additionally, MnPs improved the expression of IL-6, neurotrophic markers, and apoptotic factors. In conclusion, treatment with MnPs attenuated the oxidative stress and ER stress caused by acute compressive spinal cord trauma and restored spinal expression of neurotrophic mediators.
Keywords: apoptosis; endoplasmic reticulum stress; neuroprotection; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Hu R., Zhou J., Lou C., Luo C., Lin J., Wang X., Li X., Bian X., Li Y., Wan Q., et al. Glial scar and neuroregeneration: Histological, functional, and magnetic resonance imaging analysis in chronic spinal cord injury. J. Neurosurg. Spine. 2010;13:169–180. doi: 10.3171/2010.3.SPINE09190. - DOI - PubMed
-
- Anjum A., Yazid M.D., Fauzi Daud M., Idris J., Ng A.M.H., Selvi Naicker A., Ismail O.H.R., Athi Kumar R.K., Lokanathan Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020;20:7533. doi: 10.3390/ijms21207533. - DOI - PMC - PubMed
-
- Bergman R., Lanz O., Shell L. Acute spinal cord trauma: Mechanisms and clinical syndromes. Vet. Med. 2000;95:846–849.
-
- Khorasanizadeh M., Yousefifard M., Eskian M., Lu Y., Chalangari M., Harrop J.S., Jazayeri S.B., Seyedpour S., Khodaei B., Hosseini M., et al. Neurological recovery following traumatic spinal cord injury: A systematic review and meta-analysis. J. Neurosurg. 2019;30:683–699. doi: 10.3171/2018.10.SPINE18802. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

