Inflammasome-Mediated Neuroinflammation: A Key Driver in Alzheimer's Disease Pathogenesis
- PMID: 40427569
- PMCID: PMC12108616
- DOI: 10.3390/biom15050676
Inflammasome-Mediated Neuroinflammation: A Key Driver in Alzheimer's Disease Pathogenesis
Abstract
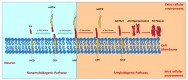
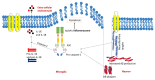
Alzheimer's disease (AD) is a progressive neurodegenerative disorder predominantly affecting the elderly, characterized by memory loss, cognitive decline, and functional impairment. While hallmark pathological features include extracellular amyloid beta (Aβ) plaques and intracellular neurofibrillary tangles composed of hyperphosphorylated tau protein, increasing evidence points to chronic neuroinflammation as a key driver of disease progression. Among inflammatory mechanisms, the activation of the NLRP3 (nucleotide-binding domain, leucine-rich repeat, and pyrin domain-containing protein 3) inflammasome in microglia plays a pivotal role by amplifying neuroinflammatory cascades, exacerbating synaptic dysfunction, and accelerating neuronal loss. This review examines the molecular underpinnings of AD with a focus on NLRP3 inflammasome-mediated neuroinflammation, detailing the crosstalk between Aβ, tau pathology, and innate immune responses. Finally, we highlight emerging therapeutic strategies targeting NLRP3 inflammasome activation as promising avenues for mitigating neuroinflammation and slowing AD progression.
Keywords: Alzheimer’s disease; NLRP3 inflammasome; amyloid beta; cytokines; microglia activation; neurodegeneration; neuroinflammation; synaptic dysfunction; tau pathology; therapeutic targets.
Conflict of interest statement
The authors affiliated with Noorda College of Osteopathic Medicine, Meritus School of Osteopathic Medicine, and Florida International University declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

