Factors Determining Kinesin Motors in a Predominant One-Head-Bound or Two-Heads-Bound State During Its Stepping Cycle
- PMID: 40427610
- PMCID: PMC12108896
- DOI: 10.3390/biom15050717
Factors Determining Kinesin Motors in a Predominant One-Head-Bound or Two-Heads-Bound State During Its Stepping Cycle
Abstract
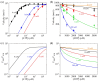
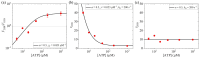
At physiological or saturating ATP concentrations, some families of kinesin motors, such as kinesin-1 and kinesin-2, exhibit a predominant two-heads-bound (2HB) state during their stepping cycle on microtubules, while others, such as kinesin-3, exhibit a predominant one-head-bound (1HB) state. An interesting but unclear issue is what factors determine a kinesin motor in the predominant 1HB and 2HB states. Here, on the basis of the general chemomechanical pathway of the kinesin motors, a theory is given on fractions of 1HB and 2HB states. With the theory, the factors affecting a kinesin motor in the predominant 1HB and 2HB states are determined. The results about the effects of ATP concentration, ADP concentration and external load on the fractions of 1HB and 2HB states are presented. Furthermore, the theory is applied to kinesin-1, kinesin-2, kinesin-3, kinesin-5 and kinesin-13 motors, with the theoretical results agreeing well with published experimental data.
Keywords: kinesin; one-head-bound state; stepping manner; two-heads-bound state.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
All-atom molecular dynamics simulations reveal how kinesin transits from one-head-bound to two-heads-bound state.Proteins. 2020 Apr;88(4):545-557. doi: 10.1002/prot.25833. Epub 2019 Oct 13. Proteins. 2020. PMID: 31589786
-
A kinetic dissection of the fast and superprocessive kinesin-3 KIF1A reveals a predominant one-head-bound state during its chemomechanical cycle.J Biol Chem. 2020 Dec 25;295(52):17889-17903. doi: 10.1074/jbc.RA120.014961. Epub 2020 Oct 20. J Biol Chem. 2020. PMID: 33082143 Free PMC article.
-
Kinesin Processivity Is Determined by a Kinetic Race from a Vulnerable One-Head-Bound State.Biophys J. 2017 Jun 20;112(12):2615-2623. doi: 10.1016/j.bpj.2017.05.007. Biophys J. 2017. PMID: 28636917 Free PMC article.
-
A model of microtubule depolymerization by kinesin-8 motor proteins.Adv Protein Chem Struct Biol. 2024;141:87-122. doi: 10.1016/bs.apcsb.2023.12.002. Epub 2023 Dec 28. Adv Protein Chem Struct Biol. 2024. PMID: 38960488 Review.
-
Kinesin motors as molecular machines.Bioessays. 2003 Dec;25(12):1212-9. doi: 10.1002/bies.10358. Bioessays. 2003. PMID: 14635256 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

