Tropomodulin-Tropomyosin Interplay Modulates Interaction Between Cardiac Myosin and Thin Filaments
- PMID: 40427620
- PMCID: PMC12109978
- DOI: 10.3390/biom15050727
Tropomodulin-Tropomyosin Interplay Modulates Interaction Between Cardiac Myosin and Thin Filaments
Abstract
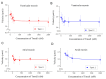
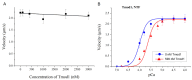
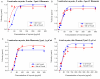
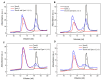
Tropomodulin (Tmod) is an actin-binding protein that interacts with tropomyosin and the actin filament at the pointed end. The influence of Tmod on the thin filament activation in the myocardium is not clear. We studied the interactions of Tmod1 and Tmod4 with the cardiac tropomyosin isoforms Tpm1.1 and Tpm1.2 using size-exclusion chromatography, a pull-down assay, and cross-linking with glutaraldehyde. We found that Tmod1 and Tmod4 form complexes with both Tpm1.1 and Tpm1.2, indicating durable interactions between these proteins. The effects of both Tmods on the actin-myosin interaction were studied using an in vitro motility assay. Tmod did not affect the sliding velocity of bare F-actin. Tmod1 slightly dose-dependently decreased the sliding velocity of F-actin-Tpm1.1 filaments and had no effect on the velocity of F-actin-Tpm1.2 filaments. With ventricular myosin, Tmod1 reduced the calcium sensitivity of the sliding velocity of thin filaments containing Tpm1.1 but did not affect it with filaments containing Tpm1.2. With atrial myosin, Tmod1 decreased the calcium sensitivity of the sliding velocities of thin filaments containing both Tpm1.1 and Tpm1.2. We can conclude that Tmod takes part in the regulation of actin-myosin interactions in the myocardium through interactions with Tpm. The effect of Tmod on the activation of thin filaments depends on the protein isoforms.
Keywords: actin-associated proteins; actin–myosin interaction; calcium regulation; cardiac myosin isoforms; cardiac tropomyosin isoforms; in vitro motility assay; tropomodulin.
Conflict of interest statement
Authors Natalia S. Ryabkova, Ivan A. Katrukha were employed by the company HyTest Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Pappas C.T., Mayfield R.M., Henderson C., Jamilpour N., Cover C., Hernandez Z., Hutchinson K.R., Chu M., Nam K.-H., Valdez J.M., et al. Knockout of Lmod2 Results in Shorter Thin Filaments Followed by Dilated Cardiomyopathy and Juvenile Lethality. Proc. Natl. Acad. Sci. USA. 2015;112:13573–13578. doi: 10.1073/pnas.1508273112. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

