LPS2336, a New TREK-1 Channel Activator Identified by High Throughput Screening
- PMID: 40427633
- PMCID: PMC12109561
- DOI: 10.3390/biom15050740
LPS2336, a New TREK-1 Channel Activator Identified by High Throughput Screening
Abstract
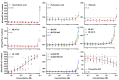
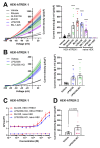
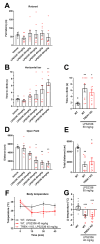
TWIK-related K+ (TREK-1) channels are involved in pain perception and their pharmacological activation has potential for pain relief. The development of new pharmacological tools to study these channels and enrich our knowledge of structure-activity relationships is therefore important. We optimized a high throughput screening method based on thallium flux monitoring for the detection of TREK-1 activators in chemical libraries. We screened 1040 compounds from the French National Essential Chemical Library and identified LPS2336 as a potent TREK-1 activator with an EC50 of 11.76 µM. Thirty-three LPS2336 analogs were subsequently tested but none of them retained activity on TREK-1. In vivo, LPS2336 produces antinociceptive activity when administered systemically and, to a lesser extent, intracerebroventricularly, but not intrathecally, showing that targeting peripheral TREK-1 channels may be important to produce pain relief, with the interest of reducing potential central adverse effects. LPS2336 was shown to produce sedation and hypothermia with a narrow therapeutic window. As these adverse effects are also observed in TREK-1 knock-out mice, they are likely mediated by off-targets. Our work provides key optimization steps for thallium-based assays and a new pharmacological tool for the study of TREK-1 channels. It also raises the importance of investigating adverse effects in vivo at early stages of drug discovery.
Keywords: TREK-1; analgesia; high throughput screening; pain; structure–activity relationship.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

