Advances in Imaging Techniques for Mammalian/Human Ciliated Cell's Cilia: Insights into Structure, Function, and Dynamics
- PMID: 40427710
- PMCID: PMC12109216
- DOI: 10.3390/biology14050521
Advances in Imaging Techniques for Mammalian/Human Ciliated Cell's Cilia: Insights into Structure, Function, and Dynamics
Abstract
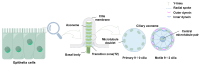
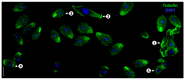
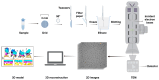
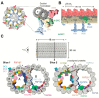
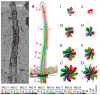
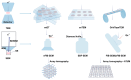
Cilia are evolutionarily conserved, microtubule-based organelles characterized by their ultrastructures and diverse functional roles, including developmental signaling, mechanosensation, and fluid propulsion. They are widely distributed across cell surfaces and play crucial roles in cell cycle regulation and tissue homeostasis. Despite advances in studying their molecular regulation and functions, demonstrating the precise ultrastructure of cilia remains a challenge. Recent novel microscopy techniques, such as super-resolution microscopy and volume electron microscopy, are revolutionizing our understanding of their architecture and mechanochemical signaling. By integrating findings from different methodologies, this review highlights how these advances bridge basic research and clinical applications and provide a comprehensive understanding of the structural organization, functional mechanisms, and dynamic changes of cilia.
Keywords: cilia; imaging techniques; volume electron microscopy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Yoke H., Taniguchi A., Nonaka S. Left-right asymmetry is formed in the basal bodies of the mouse node cilia in a cilia motility-dependent manner. bioRxiv. 2023 doi: 10.1101/2023.09.13.557556. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

