Susceptibility-Weighted Imaging (SWI): Technical Aspects and Applications in Brain MRI for Neurodegenerative Disorders
- PMID: 40428092
- PMCID: PMC12109288
- DOI: 10.3390/bioengineering12050473
Susceptibility-Weighted Imaging (SWI): Technical Aspects and Applications in Brain MRI for Neurodegenerative Disorders
Abstract
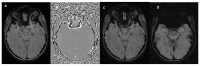
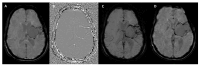
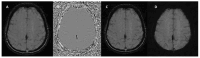
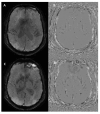
Susceptibility-weighted imaging (SWI) is a magnetic resonance imaging (MRI) sequence sensitive to substances that alter the local magnetic field, such as calcium and iron, allowing phase information to distinguish between them. SWI is a 3D gradient-echo sequence with high spatial resolution that leverages both phase and magnitude effects. The interaction of paramagnetic (such as hemosiderin and deoxyhemoglobin), diamagnetic (including calcifications and minerals), and ferromagnetic substances with the local magnetic field distorts it, leading to signal changes. Neurodegenerative diseases are typically characterized by the progressive loss of neurons and their supporting cells within the neurovascular unit. This cellular decline is associated with a corresponding deterioration of both cognitive and motor abilities. Many neurodegenerative disorders are associated with increased iron accumulation or microhemorrhages in various brain regions, making SWI a valuable diagnostic tool in clinical practice. Suggestive SWI findings are known in Parkinson's disease, Lewy body dementia, atypical parkinsonian syndromes, multiple sclerosis, cerebral amyloid angiopathy, amyotrophic lateral sclerosis, hereditary ataxias, Huntington's disease, neurodegeneration with brain iron accumulation, and chronic traumatic encephalopathy. This review will assist radiologists in understanding the technical framework of SWI sequences for a correct interpretation of currently established MRI findings and for its potential future clinical applications.
Keywords: Lewy body dementia; Parkinson disease; brain diseases; cerebral amyloid angiopathy; magnetic resonance imaging; multiple sclerosis; neurology; quantitative susceptibility mapping; radiology; susceptibility-weighted imaging.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

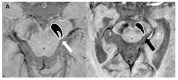
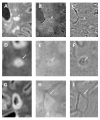
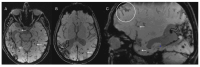

References
-
- Parillo M., Quattrocchi C., Pilato F., Di Lazzaro V., Zobel B.B. Whole-body computed tomography as first-line imaging procedure to exclude cancer in patients with neurological suspicion of paraneoplastic syndromes: Shall clinical practice adhere to recommendations? Radiography. 2023;29:8–13. doi: 10.1016/j.radi.2022.09.001. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

