Assessing Implantation Sites for Pancreatic Islet Cell Transplantation: Implications for Type 1 Diabetes Mellitus Treatment
- PMID: 40428118
- PMCID: PMC12108884
- DOI: 10.3390/bioengineering12050499
Assessing Implantation Sites for Pancreatic Islet Cell Transplantation: Implications for Type 1 Diabetes Mellitus Treatment
Abstract
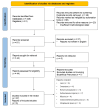
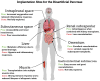
Type 1 diabetes mellitus (T1DM) involves the destruction of pancreatic β-cells, requiring ongoing insulin therapy. A promising alternative for management is pancreatic islet transplantation, or the bioartificial pancreas. Here, we examine the primary implantation sites for the bioartificial pancreas, highlighting their anatomical, physical, and immunological characteristics in the context of T1DM treatment. Traditionally used for islet transplantation, the liver promotes metabolic efficiency due to portal drainage; however, it presents issues such as hypoxia and inflammatory responses. The omentum offers excellent vascularization but has limited capacity for subsequent transplants. The renal subcapsular space is advantageous when combined with kidney transplants; however, its use is limited due to low vascularization. The subcutaneous space is notable for its accessibility and lower invasiveness, although its poor vascularization poses significant challenges. These challenges can be mitigated with bioengineering strategies. The gastrointestinal submucosa provides easy access and good vascularization, which makes it a promising option for endoscopic approaches. Additionally, the intrapleural space, which remains underexplored, offers benefits such as increased oxygenation and reduced inflammatory response. Selecting the ideal site for bioartificial pancreas implantation should balance graft support, complication reduction, and surgical accessibility. Bioengineered devices and scaffolds can address the limitations of traditional sites and enhance T1DM management.
Keywords: artificial pancreas; decellularized pancreas; diabetes mellitus; pancreatic islet transplantation; recellularized pancreas.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The Role of the Pancreatic Extracellular Matrix as a Tissue Engineering Support for the Bioartificial Pancreas.Biomimetics (Basel). 2024 Oct 2;9(10):598. doi: 10.3390/biomimetics9100598. Biomimetics (Basel). 2024. PMID: 39451804 Free PMC article. Review.
-
In Vivo Differentiation of Stem Cell-derived Human Pancreatic Progenitors to Treat Type 1 Diabetes.Stem Cell Rev Rep. 2020 Dec;16(6):1139-1155. doi: 10.1007/s12015-020-10018-5. Stem Cell Rev Rep. 2020. PMID: 32844324 Review.
-
Selection of Implantation Sites for Transplantation of Encapsulated Pancreatic Islets.Tissue Eng Part B Rev. 2018 Jun;24(3):191-214. doi: 10.1089/ten.TEB.2017.0311. Epub 2018 Jan 4. Tissue Eng Part B Rev. 2018. PMID: 29048258 Review.
-
Enhancing longevity of immunoisolated pancreatic islet grafts by modifying both the intracapsular and extracapsular environment.Acta Biomater. 2023 Sep 1;167:38-53. doi: 10.1016/j.actbio.2023.06.038. Epub 2023 Jun 29. Acta Biomater. 2023. PMID: 37392934 Review.
-
A capsule-based scaffold incorporating decellularized extracellular matrix and curcumin for islet beta cell therapy in type 1 diabetes mellitus.Biofabrication. 2024 Sep 19;16(4). doi: 10.1088/1758-5090/ad7907. Biofabrication. 2024. PMID: 39255833
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources