Development of an RT-LAMP Assay for Detecting tet(M) in Enterococcus Species: Enhancing AMR Surveillance Within the One Health Sectors
- PMID: 40428207
- PMCID: PMC12109771
- DOI: 10.3390/diagnostics15101213
Development of an RT-LAMP Assay for Detecting tet(M) in Enterococcus Species: Enhancing AMR Surveillance Within the One Health Sectors
Abstract
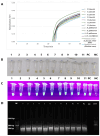
The increasing prevalence of antimicrobial-resistant (AMR) bacteria in humans, animals, and the environment underscores the necessity for a rapid, sensitive, and specific method to identify resistance genes. Objectives: This study aims to develop a reliable detection tool for identifying the tetracycline-resistant gene tet(M) in Enterococcus species using a real-time loop-mediated isothermal amplification (RT-LAMP) assay. Real-time visualization through a turbidimeter enabled precise estimation of time-to-positivity for gene detection. Methodology: Six primers were designed using PrimerExplorer v.5, and the assay was optimized across different temperatures and incubation times. Validation was conducted by testing 52 tet(M)-positive clinical enterococci isolates and spiking urine samples from a healthy volunteer and a cow with tet(M)-positive Enterococcus species. Results: The tet(M) gene was detected as early as 33 min, with optimal amplification occurring within 60 min at 60 °C. The assay demonstrated 100% specificity with the established primers. The sigmoidal graphs were corroborated with visual confirmation methods, including a green color change (visible to the naked eye), green fluorescence (under UV light), and a 200 bp PCR product observed via agarose gel electrophoresis. Notably, the tet(M) RT-LAMP assay exhibited a detection limit of 0.001 pg/μL, significantly surpassing conventional PCR, which had a detection limit of 0.1 pg/μL. Conclusions: This rapid, cost-effective, highly sensitive, and specific tet(M) RT-LAMP assay holds significant promise as a surveillance tool for antimicrobial resistance monitoring within a One Health framework, particularly in low-resource countries.
Keywords: One Health; RT-LAMP; antimicrobial-resistant; enterococci; tet(M); tetracycline resistance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Makarov D.A., Ivanova O.E., Pomazkova A.V., Egoreva M.A., Prasolova O.V., Lenev S.V., Gergel M.A., Bukova N.K., Karabanov S.Y. Antimicrobial resistance of commensal Enterococcus faecalis and Enterococcus faecium from food-producing animals in Russia. Vet. World. 2022;15:611–621. doi: 10.14202/vetworld.2022.611-621. - DOI - PMC - PubMed
-
- Iancu A.-V., Arbune M., Zaharia E.-A., Tutunaru D., Maftei N.-M., Peptine L.-D., Țocu G., Gurău G. Prevalence and Antibiotic Resistance of Enterococcus spp.: A Retrospective Study in Hospitals of Southeast Romania. Appl. Sci. 2023;13:3866. doi: 10.3390/app13063866. - DOI
LinkOut - more resources
Full Text Sources

