The Emerging Biomarkers in Chronic Obstructive Pulmonary Disease: A Narrative Review
- PMID: 40428238
- PMCID: PMC12110743
- DOI: 10.3390/diagnostics15101245
The Emerging Biomarkers in Chronic Obstructive Pulmonary Disease: A Narrative Review
Abstract
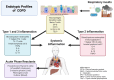
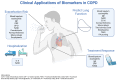
The burden of chronic obstructive pulmonary disease (COPD) is increasing, especially for women in low-to-middle income countries. Biomarkers provide ever-increasing diagnostic precision for COPD and show promise for primary, secondary, and tertiary disease prevention. This review describes emerging applications for biomarkers in COPD, especially as they align with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) emphasis on prevention, early diagnosis, and response to therapy. These biomarkers include blood eosinophils; IgE; C-reactive protein; fibrinogen; procalcitonin; interleukins 6, 8, and 33; tumor necrosis factor alpha; and soluble receptor for advanced glycated products (sRAGE). They have been used in various ways to identify COPD endotypes, predict exacerbations, predict mortality, and monitor the response to therapy. The fraction of exhaled nitric oxide (FENO) is increasingly studied in eosinophilic COPD endotypes and can be a diagnostic and predictive non-invasive biomarker. Imaging biomarkers, especially the quantitative computerized tomography (QCT) assessment of airway remolding, functional small airway disease, air trapping, lung function, and volume surrogates, all serve as non-invasive biomarkers for screening, early detection, and disease progression. Biomarkers facilitate all the phases of COPD care from detecting early airflow obstruction to predicting exacerbation and mortality. Biomarkers will be increasingly used as precise diagnostic tools to improve the COPD outcomes. The aim of this narrative review is to summarize the recent investigations in COPD biomarkers and their clinical applications.
Keywords: COPD endotypes; FENO; biomarkers; eosinophilic COPD; quantitative computerized tomography.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Correlation between fractional exhaled nitric oxide and sputum eosinophilia in exacerbations of COPD.Int J Chron Obstruct Pulmon Dis. 2017 Apr 27;12:1287-1293. doi: 10.2147/COPD.S134998. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 28490872 Free PMC article.
-
Fractional Exhaled Nitric Oxide Guided-Therapy in Chronic Obstructive Pulmonary Disease: A Stratified, Randomized, Controlled Trial.Arch Bronconeumol. 2022 Aug;58(8):601-610. doi: 10.1016/j.arbres.2021.11.013. Epub 2021 Dec 18. Arch Bronconeumol. 2022. PMID: 35312525 Clinical Trial. English, Spanish.
-
Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort.Lancet Respir Med. 2017 Dec;5(12):956-967. doi: 10.1016/S2213-2600(17)30432-0. Epub 2017 Nov 13. Lancet Respir Med. 2017. PMID: 29146301 Free PMC article.
-
Measuring exhaled nitric oxide in COPD: from theoretical consideration to practical views.Expert Rev Respir Med. 2024 Dec;18(12):1013-1024. doi: 10.1080/17476348.2024.2433537. Epub 2024 Nov 25. Expert Rev Respir Med. 2024. PMID: 39587387 Review.
-
Clinical utilization of airway inflammatory biomarkers in the prediction and monitoring of clinical outcomes in patients with chronic obstructive pulmonary disease.Expert Rev Mol Diagn. 2024 May;24(5):409-421. doi: 10.1080/14737159.2024.2344777. Epub 2024 May 17. Expert Rev Mol Diagn. 2024. PMID: 38635513 Review.
References
-
- Bhatt S.P., Rabe K.F., Hanania N.A., Vogelmeier C.F., Bafadhel M., Christenson S.A., Papi A., Singh D., Laws E., Patel N., et al. NOTUS Study Investigators. Dupilumab for COPD with Blood Eosinophil Evidence of Type 2 Inflammation. N. Engl. J. Med. 2024;390:2274–2283. doi: 10.1056/NEJMoa2401304. - DOI - PubMed
-
- Celli B.R., Anderson J.A., Brook R., Calverley P., Cowans N.J., Crim C., Dixon I., Kim V., Martinez F.J., Morris A., et al. Serum biomarkers and outcomes in patients with moderate COPD: A substudy of the randomised SUMMIT trial. BMJ Open Respir. Res. 2019;6:e000431. doi: 10.1136/bmjresp-2019-000431. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials