Functional Equivalence of Insulin and IGF-1 in the In Vitro Culture of Chicken Primordial Germ Cells
- PMID: 40428303
- PMCID: PMC12110881
- DOI: 10.3390/genes16050481
Functional Equivalence of Insulin and IGF-1 in the In Vitro Culture of Chicken Primordial Germ Cells
Abstract
Background: Chicken Primordial Germ Cells (PGCs) are one of the few germ cells that can be cultured for a long time in vitro, but challenges remain such as low culture efficiency and unclear roles of nutrient factors and signaling pathways.
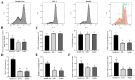
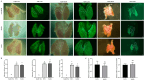
Method: In this study, protein kinase B (AKT) pathway activator insulin-like growth factor 1 (IGF-1) was screened for its ability to promote cell proliferation by transcriptome results using various inhibitors of pathway activation. The effects of IGF-1 on PGCs were evaluated through EdU assays, qRT-PCR, flow cytometry, and migration experiments.
Results: This study systematically examined the effects of insulin and IGF-1 on the proliferation, cell cycle, ferroptosis, migration capacity, and establishment efficiency of PGCs. The findings demonstrated that IGF-1 exhibited comparable effects to insulin and could effectively replace insulin in PGC culture systems.
Conclusions: The research results are expected to provide a solid theoretical basis for optimizing the chicken PGC cultivation system and promoting its practical application.
Keywords: IGF-1; chicken PGCs; establishment efficiency; migration.
Conflict of interest statement
The authors declare that this research was conducted in the absence of commercial or financial relationships that could be construed as potential conflicts of interest.
Figures




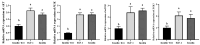


Similar articles
-
Role of PI3K/AKT signaling pathway involved in self-renewing and maintaining biological properties of chicken primordial germ cells.Poult Sci. 2024 Nov;103(11):104140. doi: 10.1016/j.psj.2024.104140. Epub 2024 Aug 8. Poult Sci. 2024. PMID: 39173217 Free PMC article.
-
[Factors influencing isolation and cloning of chicken primordial germ cells].Shi Yan Sheng Wu Xue Bao. 2004 Jun;37(3):247-50. Shi Yan Sheng Wu Xue Bao. 2004. PMID: 15323429 Chinese.
-
Chicken Primordial Germ Cells Do Not Proliferate in Insulin-Lacking Media.Int J Mol Sci. 2025 Mar 28;26(7):3122. doi: 10.3390/ijms26073122. Int J Mol Sci. 2025. PMID: 40243906 Free PMC article.
-
Effects of Insulin on Proliferation, Apoptosis, and Ferroptosis in Primordial Germ Cells via PI3K-AKT-mTOR Signaling Pathway.Genes (Basel). 2023 Oct 22;14(10):1975. doi: 10.3390/genes14101975. Genes (Basel). 2023. PMID: 37895324 Free PMC article.
-
Avian Primordial Germ Cells.Adv Exp Med Biol. 2017;1001:1-18. doi: 10.1007/978-981-10-3975-1_1. Adv Exp Med Biol. 2017. PMID: 28980226 Review.
Cited by
-
Chicken Primordial Germ Cell Surface Marker.Animals (Basel). 2025 Jun 24;15(13):1868. doi: 10.3390/ani15131868. Animals (Basel). 2025. PMID: 40646767 Free PMC article.
References
-
- Farzaneh M., Khoshnam S.E., Nokhbatolfoghahai M. First scientific record of two cases of partial twinning in the chick embryo, Gallus gallus domesticus. Vet. Rec. Case Rep. 2016;4:e000353. doi: 10.1136/vetreccr-2016-000353. - DOI
-
- Trefil P., Aumann D., Koslová A., Mucksová J., Beneová B., Kalina J., Wurmser C., Fries R., Elleder D., Schusser B. Male fertility restored by transplanting primordial germ cells into testes: A new way towards efficient transgenesis in chicken. Sci. Rep. 2017;7:14246. doi: 10.1038/s41598-017-14475-w. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

