Revisiting the Pathogenesis of X-Linked Adrenoleukodystrophy
- PMID: 40428412
- PMCID: PMC12111468
- DOI: 10.3390/genes16050590
Revisiting the Pathogenesis of X-Linked Adrenoleukodystrophy
Abstract
Background: X-ALD is a white matter (WM) disease caused by mutations in the ABCD1 gene encoding the transporter of very-long-chain fatty acids (VLCFAs) into peroxisomes. Strikingly, the same ABCD1 mutation causes either devastating brain inflammatory demyelination during childhood or, more often, progressive spinal cord axonopathy starting in middle-aged adults. The accumulation of undegraded VLCFA in glial cell membranes and myelin has long been thought to be the central mechanism of X-ALD.
Methods: This review discusses studies in mouse and drosophila models that have modified our views of X-ALD pathogenesis.
Results: In the Abcd1 knockout (KO) mouse that mimics the spinal cord disease, the late manifestations of axonopathy are rapidly reversed by ABCD1 gene transfer into spinal cord oligodendrocytes (OLs). In a peroxin-5 KO mouse model, the selective impairment of peroxisomal biogenesis in OLs achieves an almost perfect phenocopy of cerebral ALD. A drosophila knockout model revealed that VLCFA accumulation in glial myelinating cells causes the production of a toxic lipid able to poison axons and activate inflammatory cells. Other mouse models showed the critical role of OLs in providing energy substrates to axons. In addition, studies on microglial changing substates have improved our understanding of neuroinflammation.
Conclusions: Animal models supporting a primary role of OLs and axonal pathology and a secondary role of microglia allow us to revisit of X-ALD mechanisms. Beyond ABCD1 mutations, pathogenesis depends on unidentified contributors, such as genetic background, cell-specific epigenomics, potential environmental triggers, and stochasticity of crosstalk between multiple cell types among billions of glial cells and neurons.
Keywords: VLCFA; X-adrenoleukodystrophy; cerebral demyelination; neuroinflammation; oligodendrocytes; peroxisomes; spinal cord axonopathy.
Conflict of interest statement
PB is the founder of the TherapyDesignConsulting biotech company.
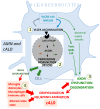
Figures

Similar articles
-
Abcd1 deficiency accelerates cuprizone-induced oligodendrocyte loss and axonopathy in a demyelinating mouse model of X-linked adrenoleukodystrophy.Acta Neuropathol Commun. 2023 Jun 18;11(1):98. doi: 10.1186/s40478-023-01595-w. Acta Neuropathol Commun. 2023. PMID: 37331971 Free PMC article.
-
HDAC inhibitor SAHA normalizes the levels of VLCFAs in human skin fibroblasts from X-ALD patients and downregulates the expression of proinflammatory cytokines in Abcd1/2-silenced mouse astrocytes.J Lipid Res. 2011 Nov;52(11):2056-69. doi: 10.1194/jlr.M017491. Epub 2011 Sep 4. J Lipid Res. 2011. PMID: 21891797 Free PMC article.
-
Lack of adrenoleukodystrophy protein enhances oligodendrocyte disturbance and microglia activation in mice with combined Abcd1/Mag deficiency.Acta Neuropathol. 2007 Dec;114(6):573-86. doi: 10.1007/s00401-007-0288-4. Epub 2007 Sep 9. Acta Neuropathol. 2007. PMID: 17828604
-
ABCD1 and X-linked adrenoleukodystrophy: A disease with a markedly variable phenotype showing conserved neurobiology in animal models.J Neurosci Res. 2021 Dec;99(12):3170-3181. doi: 10.1002/jnr.24953. Epub 2021 Oct 29. J Neurosci Res. 2021. PMID: 34716609 Free PMC article. Review.
-
ABC subfamily D proteins and very long chain fatty acid metabolism as novel targets in adrenoleukodystrophy.Curr Drug Targets. 2011 May;12(5):694-706. doi: 10.2174/138945011795378577. Curr Drug Targets. 2011. PMID: 21039332 Review.
Cited by
-
The neurological pathology of peroxisomal ACBD5 deficiency - lessons from patients and mouse models.Front Mol Neurosci. 2025 Jul 2;18:1602343. doi: 10.3389/fnmol.2025.1602343. eCollection 2025. Front Mol Neurosci. 2025. PMID: 40672445 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

