Antioxidant Peptides from the Fruit Source of the Oil Crop Litsea cubeba Ameliorate FFA-Induced Oxidative Stress Injury: Based on Nrf2/Keap1 Pathway and Molecular Dynamics Simulations
- PMID: 40428488
- PMCID: PMC12111390
- DOI: 10.3390/foods14101707
Antioxidant Peptides from the Fruit Source of the Oil Crop Litsea cubeba Ameliorate FFA-Induced Oxidative Stress Injury: Based on Nrf2/Keap1 Pathway and Molecular Dynamics Simulations
Abstract
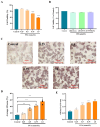
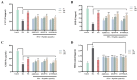
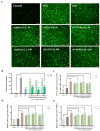
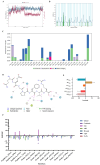
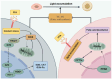
In this study, we systematically investigated the mechanisms of the antioxidation and anti-lipid accumulation effects of antioxidant peptides from Litsea cubeba on a free fatty acid (FFA)-induced NAFLD model of HepG2 cells. The NAFLD cell model was constructed by inducing the HepG2 hepatocellular carcinoma cell line with 0.5 mmol/L FFAs, and AQRDAGLL, QEGPFVR, and DVPPPRGPL were given to the culture to study their lipid-lowering and antioxidant activities on NAFLD cells. The lipid-lowering activities of the three antioxidant peptides were evaluated by Oil Red O staining and TG and TC content assays, and the results showed that all three peptides had strong ameliorating effects on FFA-induced lipid accumulation in NAFLD cells. The intracellular antioxidant protease (CAT, GSH, and SOD) activity levels and lipid peroxidation (MDA) content were measured and intracellular ROS levels were detected. The results showed that after intervention with the antioxidant peptides, the intracellular ROS levels in the NAFLD model cells were significantly reduced, the SOD and CAT activities were increased, the GSH content was elevated, and the MDA content was reduced, which indicated that AQRDAGLL, QEGPFVR, and DVPPPRGPL were able to inhibit the oxidative stress of the cells effectively and to achieve the effect of intervening in NAFLD. JC-1 fluorescence staining experiments showed that the mitochondrial membrane potential function of NAFLD cells was restored under the effect of the antioxidant peptides. Molecular dynamics simulations revealed that the main driving force between QEGPFVR and Keap1 protein was van der Waals forces, ΔG = -62.11 kcal/mol, which indicated that QEGPFVR was capable of spontaneously binding to Keap1 protein.
Keywords: Keap1/Nrf2 pathway; Litsea cubeba antioxidant peptides; ROS; molecular docking; molecular dynamics simulations.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures





References
-
- Ahmad T., Belwal T., Li L., Ramola S., Aadil R.M., Abdullah, Xu Y., Zisheng L. Utilization of wastewater from edible oil industry, turning waste into valuable products: A review. Trends Food Sci. Technol. 2020;99:21–33. doi: 10.1016/j.tifs.2020.02.017. - DOI
-
- Xu L., Wei Z., Guo B., Bai R., Liu J., Li Y., Sun W., Jiang X., Li X., Pi Y. Flaxseed Meal and Its Application in Animal Husbandry: A Review. Agriculture. 2022;12:2027. doi: 10.3390/agriculture12122027. - DOI
-
- Yong K.J., Wu T.Y. Second-generation bioenergy from oilseed crop residues: Recent technologies, techno-economic assessments and policies. Energy Convers. Manag. 2022;267:115869. doi: 10.1016/j.enconman.2022.115869. - DOI
-
- Kotecka-Majchrzak K., Sumara A., Fornal E., Montowska M. Oilseed proteins—Properties and application as a food ingredient. Trends Food Sci. Technol. 2020;106:160–170. doi: 10.1016/j.tifs.2020.10.004. - DOI
-
- Shao J., Zhang G., Fu J., Zhang B. Advancement of the preparation methods and biological activity of peptides from sesame oil byproducts: A review. Int. J. Food Prop. 2020;23:2189–2200. doi: 10.1080/10942912.2020.1849276. - DOI
Grants and funding
- 20203ABC28W016/The Major Science and Technology Research & Development Special Project
- 2020CXZX07/Special Research Project on Camphor Tree (KRPCT) of Jiangxi Forestry Department
- YC2023-B136/Jiangxi Province Graduate Innovation Special Fund Project
- 202310410006/College Students' Innovative Entrepreneurial Training Plan Program
LinkOut - more resources
Full Text Sources
Miscellaneous

