A Comparative Morphological Study of the Ultrastructure of Antennal Sensilla in Sclerodermus guani (Hymenoptera: Bethylidae)
- PMID: 40429260
- PMCID: PMC12112000
- DOI: 10.3390/insects16050547
A Comparative Morphological Study of the Ultrastructure of Antennal Sensilla in Sclerodermus guani (Hymenoptera: Bethylidae)
Abstract
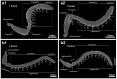
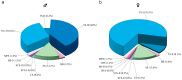
The morphology, number, and distribution of antennal sensilla differ between males and females, reflecting adaptations to sex-specific ecological roles and life histories. In this study, scanning electron microscopy was employed to examine the antennal structure and sensilla types of adult males and females of Sclerodermus guani Xiao et Wu 1983 (Hymenoptera: Bethylidae), with a focus on identifying morphological differences between the sexes. The results revealed that the antennae of both sexes are geniculate; however, female antennae are shorter and broader than those of males. Each antenna comprises 13 segments, including a scape (1 segment), a pedicel (1 segment), and a flagellum (11 segments). Eight distinct types of sensilla were identified on the antennae of both males and females, with notable sex-specific differences in sensilla types and subtypes. Trichoid sensilla subtype III was found exclusively in males, whereas long basiconic sensilla and basiconic sensilla subtype II were unique to females. More than 70% of the antennal sensilla in both sexes were olfactory in nature, highlighting their predominant role in chemical detection. The observed sexual dimorphism in the morphology and distribution of olfactory sensilla suggests functional specialization, potentially linked to host localization in females and mate location in males.
Keywords: Sclerodermus guani; antennae; morphology; olfactory sensilla; scanning electron microscope.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Antennal sensilla in the parasitoid Sclerodermus sp. (Hymenoptera: Bethylidae).J Insect Sci. 2015 Apr 5;15(1):36. doi: 10.1093/jisesa/iev024. Print 2015. J Insect Sci. 2015. PMID: 25843589 Free PMC article.
-
Morphology and ultrastructure of antennal sensilla of the parasitic wasp Baryscapus dioryctriae (Hymenoptera: Eulophidae).Microsc Res Tech. 2023 Jan;86(1):12-27. doi: 10.1002/jemt.24253. Epub 2022 Nov 1. Microsc Res Tech. 2023. PMID: 36318186
-
Antennal chemoreceptors in the European ectoparasitoid Sclerodermus cereicollis (Hymenoptera: Bethylidae).Microsc Res Tech. 2024 Oct;87(10):2275-2291. doi: 10.1002/jemt.24597. Epub 2024 May 11. Microsc Res Tech. 2024. PMID: 38733292
-
Morphology and distribution of antennal sensilla in the gall midge Gephyraulus lycantha (Diptera: Cecidomyiidae).Micron. 2021 Jun;145:103061. doi: 10.1016/j.micron.2021.103061. Epub 2021 Mar 19. Micron. 2021. PMID: 33773439
-
Ultrastructure and distribution of antennal sensilla of parasitic wasp, Cotesia gregalis (Hymenoptera: Braconidae).Microsc Res Tech. 2024 Dec;87(12):2954-2963. doi: 10.1002/jemt.24666. Epub 2024 Jul 27. Microsc Res Tech. 2024. PMID: 39072964
References
-
- Das P., Chen L., Sharma K.R., Fadamiro H.Y. Abundance of antennal chemosensilla in two parasitoid wasps with different degree of host specificity may explain sexual and species differences in their response to host-related volatiles. Microsc. Res. Tech. 2011;74:900–909. doi: 10.1002/jemt.20974. - DOI - PubMed
-
- Nowińska A., Brożek J. Antennal sensory structures in water bugs of Nepoidea (Insecta: Hemiptera: Nepomorpha), their morphology and function. Zoomorphology. 2019;138:307–319. doi: 10.1007/s00435-019-00446-4. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

