Characterisation of Mesenchymal Stromal Cells (MSCs) from Human Adult Thymus as a Potential Cell Source for Regenerative Medicine
- PMID: 40429470
- PMCID: PMC12112012
- DOI: 10.3390/jcm14103474
Characterisation of Mesenchymal Stromal Cells (MSCs) from Human Adult Thymus as a Potential Cell Source for Regenerative Medicine
Abstract
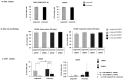
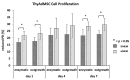
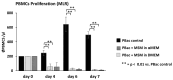
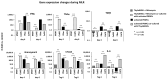
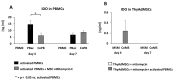
Background: Mesenchymal stem cell-based therapy may be indicated in ischaemic heart disease. The use of autologous adipose-derived mesenchymal stromal cells (AdMSCs) offers regenerative potential due to their paracrine effects. The aim of this study was to expand and characterise adult human thymus-derived MSCs harvested during open heart surgery with respect to their stem cell and paracrine properties. Methods: Enzymatically and non-enzymatically isolated human thymic AdMSCs (ThyAdMSCs) were cultured in xeno-free media containing pooled human platelet lysate (pPL). MSC characterisation was performed. Ex vivo expanded ThyAdMSCs were differentiated into three lineages. Proliferative capacity and immunomodulatory properties were assessed by proliferation assays and mixed lymphocyte reaction, respectively. Gene expression analysis was performed by qPCR. Results: Both isolation methods yielded fibroblast-like cells with plastic adherence and high proliferation. Flow cytometry revealed distinct expression of MSC markers in the absence of haematopoietic cell surface markers. Ex vivo expanded ThyAdMSCs could be differentiated into adipocytes, osteocytes, and chondrocytes. Activated peripheral blood mononuclear cells were significantly reduced when co-cultured with ThyAdMSCs, indicating their ability to inhibit immune cells in vitro. Gene expression analysis showed significantly less IFNγ and TNFα, indicating an alteration of the activated and pro-inflammatory state in the presence of ThyAdMSCs. Conclusions: These results demonstrate an efficient method to generate AdMSCs from human thymus. These MSCs have a strong immunomodulatory capacity and are, therefore, a promising cell source for regenerative medicine. The culture conditions are crucial for cells to proliferate in culture. Further research could explore the use of ThyAdMSCs or their secretome in surgical procedures.
Keywords: adipose tissue; adult thymus; cardiac surgery; cell therapy; mesenchymal stromal cell; platelet lysate.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Regulatory-compliant conditions during cell product manufacturing enhance in vitro immunomodulatory properties of infrapatellar fat pad-derived mesenchymal stem/stromal cells.Cytotherapy. 2020 Nov;22(11):677-689. doi: 10.1016/j.jcyt.2020.06.007. Epub 2020 Jul 26. Cytotherapy. 2020. PMID: 32723596
-
Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy.Stem Cell Res Ther. 2015 Apr 13;6(1):55. doi: 10.1186/s13287-015-0066-5. Stem Cell Res Ther. 2015. PMID: 25884704 Free PMC article.
-
The roles of regulatory-compliant media and inflammatory/oxytocin priming selection in enhancing human mesenchymal stem/stromal cell immunomodulatory properties.Sci Rep. 2024 Nov 27;14(1):29438. doi: 10.1038/s41598-024-80050-9. Sci Rep. 2024. PMID: 39604514 Free PMC article.
-
Mesenchymal stem cell secretome and regenerative therapy after cancer.Biochimie. 2013 Dec;95(12):2235-45. doi: 10.1016/j.biochi.2013.05.010. Epub 2013 Jun 5. Biochimie. 2013. PMID: 23747841 Free PMC article. Review.
-
Regenerative medicine strategies for hair growth and regeneration: A narrative review of literature.Regen Ther. 2022 Oct 31;21:527-539. doi: 10.1016/j.reth.2022.10.005. eCollection 2022 Dec. Regen Ther. 2022. PMID: 36382136 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

