Osilodrostat Safety Profile: Findings from Real-World Data in the FAERS Database
- PMID: 40429513
- PMCID: PMC12112102
- DOI: 10.3390/jcm14103518
Osilodrostat Safety Profile: Findings from Real-World Data in the FAERS Database
Abstract
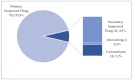
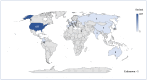
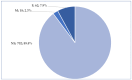
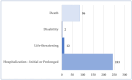
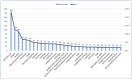
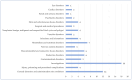
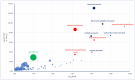
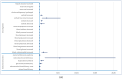
Background/Objectives: Cushing's syndrome (CS), including Cushing's disease (CD)-the most common type-has a substantial negative impact on morbidity, mortality, and patients' quality of life. Medical management of CS is essential for controlling hypercortisolism as part of preoperative preparation for definitive surgical treatment and for managing residual or relapsed hypercortisolism post-surgery. Osilodrostat, a dual inhibitor of glucocorticoid and mineralocorticoid biosynthetic pathways, has been approved for the medical treatment of CS since early 2020. However, real-world data on its adverse effects remain limited. We mined the FAERS database and analyzed the reports associated with osilodrostat up to 1 October 2024. Methods: Descriptive and disproportionality methods based on Relative Odds Ratio (ROR), Chi-square (χ2), and Proportional Reporting Ratio (PRR), were used to discern potential safety signals and assess the significance of osilodrostat-associated adverse events. Results: This study identified 782 reports in which osilodrostat was the primary suspected drug, containing 593 preferred terms (PTs) and 2481 occurrences. The most frequently registered events belonged to the following SOCs: "General disorders and administration site conditions" (n = 457, 18.4%), "Injury, poisoning and procedural complications" (n = 311, 12.5%), "Gastrointestinal disorders" (n = 278, 11.2%), "Investigations" (n = 260, 10.5%), and "Nervous system disorders" (n = 184, 7.4%). Among PTs, off-label use was the most commonly reported, aligning with the fact that the vast majority of cases originated from the U.S. (84%), where osilodrostat is officially approved only for the treatment of CD. Disproportionality analysis confirmed previously known and new potential adverse drug reactions associated with osilodrostat treatment, including reports of cardiac flutter (n: 4; PRR: 19.42; χ2: 49.57), ventricular extrasystoles (n: 4; PRR: 11.85; χ2: 29.62), muscular weakness (n: 8; PRR: 2.25; χ2: 4.38), rib fracture (n: 4; PRR: 6.66; χ2: 13.99), spinal fracture (n: 3; PRR: 4.66; χ2: 5.35), sepsis (n: 9; PRR: 2.63; χ2: 7.56), fungal infections (n: 4; PRR: 3.67; χ2: 5.33), and COVID-19 (n: 32; PRR: 5.07; χ2: 101.16). Conclusions: This study highlights new risks and offers valuable insights into osilodrostat use; however, further research and validation are necessary, particularly for adverse reactions not yet explicitly documented in the summary of product characteristics.
Keywords: FAERS database; adverse effect; disproportionality analysis; osilodrostat; pharmacovigilance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Suspected adverse drug reactions of rivaroxaban reported in the United States food and drug administration adverse event reporting system database: a pharmacovigilance study.Front Pharmacol. 2024 Sep 6;15:1399172. doi: 10.3389/fphar.2024.1399172. eCollection 2024. Front Pharmacol. 2024. PMID: 39309013 Free PMC article.
-
Post-market safety profile of cefiderocol: a real-world pharmacovigilance exploratory analysis based on U.S. FDA adverse event reporting system (FAERS).BMC Pharmacol Toxicol. 2025 Mar 11;26(1):58. doi: 10.1186/s40360-025-00894-3. BMC Pharmacol Toxicol. 2025. PMID: 40069825 Free PMC article.
-
Post-marketing safety profile of ganirelix in women: a 20-year pharmacovigilance analysis of global adverse drug event databases (2004-2024).BMC Pharmacol Toxicol. 2025 Apr 22;26(1):91. doi: 10.1186/s40360-025-00920-4. BMC Pharmacol Toxicol. 2025. PMID: 40264185 Free PMC article.
-
Treatment of Cushing's syndrome with osilodrostat: practical applications of recent studies with case examples.Pituitary. 2022 Dec;25(6):795-809. doi: 10.1007/s11102-022-01268-2. Epub 2022 Aug 24. Pituitary. 2022. PMID: 36002784 Free PMC article. Review.
-
Romosozumab adverse event profile: a pharmacovigilance analysis based on the FDA Adverse Event Reporting System (FAERS) from 2019 to 2023.Aging Clin Exp Res. 2025 Jan 14;37(1):23. doi: 10.1007/s40520-024-02921-5. Aging Clin Exp Res. 2025. PMID: 39808360 Free PMC article.
References
LinkOut - more resources
Full Text Sources

