Evidence for the Necessity of Objective Hearing Tests in Cochlear Implantation Assessment: Excluding Functional Hearing Loss Cases
- PMID: 40429580
- PMCID: PMC12112472
- DOI: 10.3390/jcm14103585
Evidence for the Necessity of Objective Hearing Tests in Cochlear Implantation Assessment: Excluding Functional Hearing Loss Cases
Abstract
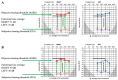
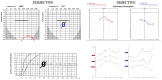
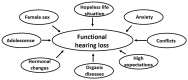
Background/Objectives: Cochlear implantation is a crucial intervention for individuals with severe hearing loss, aiming to restore auditory function and improve quality of life. The decision to recommend cochlear implantation critically depends on accurate audiological evaluations. However, challenges arise when subjective assessments of hearing loss do not align with objective audiological measurements, leading to potential misdiagnoses. Comparisons are to be made between subjective and objective results, with an investigation into the characteristics, warning signs, and risk factors of functional hearing loss (FHL). Methods: A retrospective study of hearing loss presentations at an otorhinolaryngological university clinic between 2020 and 2024 was performed, whereby we collected FHL cases. The evaluation process included measurements of subjectively perceived hearing loss through pure-tone audiometry, speech understanding, and communication testing. The objective assessments comprised impedance measurement, otoacoustic emission measurement, auditory brainstem responses, auditory steady-state responses, and medical imaging. Results: During the studied period, 11 patients, with an average age of 35.2 years (13 to 64 years), who were originally referred for cochlear implantation evaluation and subsequently diagnosed with FHL, were identified. The majority (10 patients) were female. No organic cause was identified in four cases, while seven cases exhibited some organic ear abnormalities insufficient to justify the reported hearing loss. The degree of FHL ranged from 30 dB to 90 dB, with an average of 60 dB. Conclusions: Diagnosing FHL is challenging and requires comprehensive assessment and interdisciplinary collaboration. Failure to recognize it may lead to inappropriate treatment, including unnecessary cochlear implantation. This study advocates for the mandatory integration of ABR and ASSR in the clinical evaluation of all cochlear implant candidates to ensure accurate diagnosis and optimal treatment.
Keywords: ABR; ASSR; assessment; cochlear implant; functional hearing loss; rehabilitation; risk factors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Evaluation and therapy outcome in children with auditory neuropathy spectrum disorder (ANSD).Int J Pediatr Otorhinolaryngol. 2019 Dec;127:109681. doi: 10.1016/j.ijporl.2019.109681. Epub 2019 Sep 13. Int J Pediatr Otorhinolaryngol. 2019. PMID: 31542652
-
Relationship Between Objective and Behavioral Audiology for Young Children Being Assessed for Cochlear Implantation: Implications for CI Candidacy Assessment.Otol Neurotol. 2019 Mar;40(3):e252-e259. doi: 10.1097/MAO.0000000000002125. Otol Neurotol. 2019. PMID: 30741904
-
Implantable Devices for Single-Sided Deafness and Conductive or Mixed Hearing Loss: A Health Technology Assessment.Ont Health Technol Assess Ser. 2020 Mar 6;20(1):1-165. eCollection 2020. Ont Health Technol Assess Ser. 2020. PMID: 32194878 Free PMC article.
-
Are auditory steady-state responses a good tool prior to pediatric cochlear implantation?Int J Pediatr Otorhinolaryngol. 2015 Aug;79(8):1257-62. doi: 10.1016/j.ijporl.2015.05.026. Epub 2015 May 28. Int J Pediatr Otorhinolaryngol. 2015. PMID: 26092547
-
[Auditory neuropathy: clinical presentation of seven cases and review of the literature].Ann Otolaryngol Chir Cervicofac. 2005 Dec;122(6):303-14. doi: 10.1016/s0003-438x(05)82365-5. Ann Otolaryngol Chir Cervicofac. 2005. PMID: 16505781 Review. French.
References
-
- American Academy of Audiology Clinical Practice Guideline: Cochlear Implants. 2019. [(accessed on 1 July 2019)]. Available online: https://www.audiology.org/wp-content/uploads/2021/05/CochlearImplantPrac....
LinkOut - more resources
Full Text Sources
Miscellaneous

