Beyond Trypanosoma cruzi: LINE-1 Activation as a Driver of Chronic Inflammation in Chagas Disease
- PMID: 40429613
- PMCID: PMC12111687
- DOI: 10.3390/ijms26104466
Beyond Trypanosoma cruzi: LINE-1 Activation as a Driver of Chronic Inflammation in Chagas Disease
Abstract
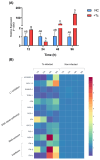
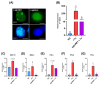
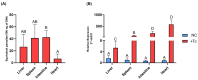
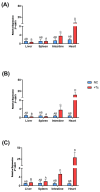
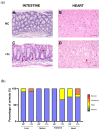
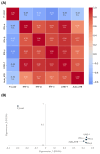
Chagas disease (CD) is endemic in Latin America, with its pathogenesis linked to Trypanosoma cruzi (Tc) persistence and autoimmune responses. This study investigates the role of LINE-1 (L1) activation in inflammation and loss of self-tolerance during Tc infection. In vitro assays evaluated the expression of genes involved in L1 regulation and interferon signaling under basal conditions and following L1 suppression via CRISPR/dCas9. In vivo analyses in a murine model included L1 and IFN expression profiling, autoantibody quantification, and histopathological assessments of liver, spleen, intestine, and heart. Tc infection induced L1 upregulation, correlating with an increased expression of its inhibitors, MOV-10 and APOBEC-3, suggesting host-driven regulatory mechanisms. L1 activation was also associated with the upregulation of DNA repair pathways (MMR and NHEJ) and RNA-sensing pathways (MDA-5 and RIG-I), leading to type I interferon responses. In the murine model, L1 expression was highest in the intestine and heart, independent of parasite burden, and correlated with increased interferon gene expression and autoantibody production. Our findings suggest that CD pathogenesis involves L1-induced chronic inflammation, which may contribute to late-stage symptoms. This highlights self-recognition mechanisms in disease severity and reveals potential therapeutic targets for novel treatments.
Keywords: Chagas disease; LINE-1 retroelement; Trypanosoma cruzi; autoimmune response; inflammation; interferons.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- World Health Organization (WHO) Chagas Disease (American Trypanosomiasis) WHO; Geneva, Switzerland: 2024. [(accessed on 17 March 2025)]. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(america...
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

