Exploring the Histopathological Features of Thrombus-Associated Localized Amyloid Deposition: Comprehensive Analysis Employing Immunohistochemistry and Proteomics
- PMID: 40429650
- PMCID: PMC12111168
- DOI: 10.3390/ijms26104505
Exploring the Histopathological Features of Thrombus-Associated Localized Amyloid Deposition: Comprehensive Analysis Employing Immunohistochemistry and Proteomics
Abstract
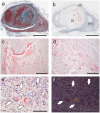
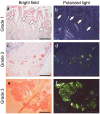
Amyloid deposition has been reported to localize within thrombi; however, its pathological characteristics, particularly its precursor proteins, remain poorly understood. This study aimed to elucidate the pathological features of thrombus-associated amyloid deposition by immunohistochemistry combined with proteomic analyses using liquid chromatography-tandem mass spectrometry with laser microdissection. Our findings revealed that thrombus-associated amyloid deposits within the thrombus and vessel wall primarily comprised apolipoprotein A-I, with a mixture of amyloid fibrils derived from amyloidogenic proteins, including transthyretin and lactoferrin. Given that these proteins are present in the blood, our results support a previous hypothesis that proteins denatured during thrombus aging are a source of amyloid. Furthermore, phagocytes were infiltrated around the intramural and extravascular deposits rather than around the amyloid deposits within the thrombus. Therefore, amyloid deposits generated within the thrombus may be transported from regions with limited blood flow to the vessel wall and surrounding tissues, where blood flow is present, during thrombus processing. These deposits were primarily removed by phagocytic cells. Our results suggest that a facilitative effect on deposition occurs via a cross-seeding mechanism between amyloid fibrils and that phagocytes can remove amyloid deposits. These findings help elucidate the pathogenesis of localized amyloidosis.
Keywords: apolipoprotein A-I; atherosclerosis; lactoferrin; localized amyloidosis; phagocyte; proteomics; thrombus; transthyretin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Buxbaum J.N., Eisenberg D.S., Fandrich M., McPhail E.D., Merlini G., Saraiva M.J.M., Sekijima Y., Westermark P. Amyloid nomenclature 2024: Update, novel proteins, and recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid. 2024;31:249–256. doi: 10.1080/13506129.2024.2405948. - DOI - PubMed
-
- Ichimata S., Hata Y., Yoshinaga T., Katoh N., Kametani F., Yazaki M., Sekijima Y., Nishida N. Amyloid-forming corpora amylacea and spheroid-type amyloid deposition: Comprehensive analysis using immunohistochemistry, proteomics, and a literature review. Int. J. Mol. Sci. 2024;25:4040. doi: 10.3390/ijms25074040. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

