Peroxisome Proliferator-Activated Receptors (PPARs) May Mediate the Neuroactive Effects of Probiotic Metabolites: An In Silico Approach
- PMID: 40429654
- PMCID: PMC12111801
- DOI: 10.3390/ijms26104507
Peroxisome Proliferator-Activated Receptors (PPARs) May Mediate the Neuroactive Effects of Probiotic Metabolites: An In Silico Approach
Abstract
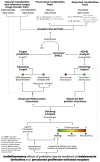
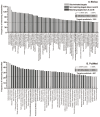
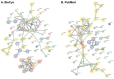
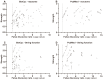
It is well established that the gut-brain axis (GBA) is a bidirectional communication between the gut and the brain. This axis, critical in maintaining overall homeostasis, is regulated at the neuronal, endocrine, and immunological levels, all of which may be influenced by the gut microbiota (GM). Therefore, dysbiosis or disruption in the GM may have serious consequences including neuroinflammation due to overactivation of the immune system. Strategies to reestablish GM integrity via use of probiotics are being pursued as novel therapeutic intervention in a variety of central and peripheral diseases. The mechanisms leading to dysbiosis or efficacy of probiotics, however, are not fully evident. Here, we performed computational analysis on two major probiotics, namely Lactobacillus Lacticaseibacillus rhamnosus GG (formerly named Lactobacillus rhamnosus, L. rhamnosus GG) and Bifidobacterium animalis spp. lactis (B. lactis or B. animalis) to not only shed some light on their mechanism(s) of action but also to identify potential molecular targets for novel probiotics. Using the PubMed web page and BioCyc Database Collection platform we specifically analyzed proteins affected by metabolites of these bacteria. Our results indicate that peroxisome proliferator-activated receptors (PPARs), nuclear receptor proteins that are involved in regulation of inflammation are key mediators of the neuroactive effect of probiotics.
Keywords: PPARs; gut microbiota; gut-brain axis; metabolites; neuroinflammation; neuroprotection; probiotics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Restitution of gut microbiota in Ugandan children administered with probiotics (Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12) during treatment for severe acute malnutrition.Gut Microbes. 2020 Jul 3;11(4):855-867. doi: 10.1080/19490976.2020.1712982. Epub 2020 Jan 20. Gut Microbes. 2020. PMID: 31959047 Free PMC article. Clinical Trial.
-
The effect of early probiotic exposure on the preterm infant gut microbiome development.Gut Microbes. 2021 Jan-Dec;13(1):1951113. doi: 10.1080/19490976.2021.1951113. Gut Microbes. 2021. PMID: 34264803 Free PMC article.
-
Synergistic Effect of Formate and Cell-Free Supernatant Fermented by Two Probiotics, Bifidobacterium animalis spp. lactis HN019 and Lacticaseibacillus rhamnosus HN001, against Shigella flexneri Growth.ACS Infect Dis. 2025 Jun 13;11(6):1424-1436. doi: 10.1021/acsinfecdis.4c00796. Epub 2025 May 15. ACS Infect Dis. 2025. PMID: 40371449
-
Health benefits of Lactobacillus rhamnosus GG and Bifidobacterium animalis subspecies lactis BB-12 in children.Postgrad Med. 2020 Jun;132(5):441-451. doi: 10.1080/00325481.2020.1731214. Epub 2020 Feb 26. Postgrad Med. 2020. PMID: 32059116 Review.
-
Mechanism of action of gut microbiota and probiotic Lactobacillus rhamnosus GG on skeletal remodeling in mice.Endocrinol Diabetes Metab. 2024 Jan;7(1):e440. doi: 10.1002/edm2.440. Epub 2023 Jul 28. Endocrinol Diabetes Metab. 2024. PMID: 37505196 Free PMC article. Review.
References
-
- Kolzhetsov N., Markelova N., Frolova M., Alikina O., Glazunova O., Safonova L., Kalashnikova I., Yudin V., Makarov V., Keskinov A., et al. Enterotype-Dependent Probiotic-Mediated Changes in the Male Rat Intestinal Microbiome In Vivo and In Vitro. Int. J. Mol. Sci. 2024;25:4558. doi: 10.3390/ijms25084558. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

