Clinical Validation of Plasma Metabolite Markers for Early Lung Cancer Detection
- PMID: 40429664
- PMCID: PMC12111207
- DOI: 10.3390/ijms26104519
Clinical Validation of Plasma Metabolite Markers for Early Lung Cancer Detection
Abstract
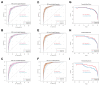
Early detection of lung cancer significantly improves survival, yet current screening methods have limitations. This study aimed to identify a robust panel of plasma metabolites for early-stage non-small cell lung cancer (NSCLC) diagnosis using a large, clinically diverse patient cohort. A total of 680 archived plasma samples from biopsy-confirmed NSCLC patients and controls (including healthy individuals and patients with non-cancerous lung diseases) were analyzed using targeted, quantitative mass spectrometry-based metabolomics and used as the discovery cohort. An independent set of 216 plasma samples served as the validation cohort. Logistic regression (LR) models developed from the discovery set using ten metabolites achieved area under the receiver-operating characteristic curve (AUROC) values of 93.63%, 93.74%, and 93.91% for distinguishing all-stage, stage I-II, and stage I NSCLC patients from controls, respectively. Incorporating smoking history further improved model performance. The validation cohort confirmed the model's robustness, demonstrating high sensitivity and specificity for early-stage detection. These results support the potential of metabolomic biomarkers as a minimally invasive, accurate tool for early NSCLC diagnosis. This approach may complement current screening methods, enabling earlier intervention and improved patient outcomes. Further studies are warranted to validate these findings in more diverse populations and real-world clinical settings.
Keywords: early detection; metabolite markers; non-small cell lung cancer.
Conflict of interest statement
R.A.B. is President and CEO of BioMark Diagnostics Inc. and is a shareholder. GH is President of BioMark Diagnostic Solutions Inc. J.-F.H. is Executive Director of BioMark Diagnostic Solutions Inc. P.S.T. is a minor shareholder of BioMark Diagnostics, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. C.D.R reports personal fees for advisory board membership from Novocure; institutional fees for advisory board membership from AstraZeneca, Imagene, MedStar, Amgem, Boeringer-Ingelheim, Hoffmann-La Roche Ltd, Janssen Pharmaceutical, NeoGenomics, Pfizer, Inc. and Regeneron; Research collaboration non remunerated: Guardant, Foundation One; institutional fees as an invited speaker from COR2ED, HPM education IDEOlogy Merck and Roche, OneCell Dx; non-renumerated leadership roles as a scientific board member for the European School of Oncology (ESO), Past Chair on the educational committee for the International Association for Study of Lung Cancer (IASLC), President for the International Society of Liquid Biopsy (ISLB) and Educational Chair for the Oncology Latin American Association (OLA); a renumerated role as Editor-in-Chief for Critical Reviews in Oncology Hematology (CROH); a non-renumerated role as ESMO Faculty Group/Speciality and Faculty Coordinator for metastatic non-small cell lung cancer for European Society for Medical Oncology (ESMO); non-renumerated roles as: Scientific Board Member at ESO (European School of Oncology), External advisor Board member of Centro Pfizer-Universidad de Granada-Junta de Andalucía de Genómica e Investigación Oncológica (GENYO), External Advisor of the School of Public Health, University of Granada, Spain, and non-renumerated analysis of liquid biopsies in a lung cancer trial for Guardant Health.
Figures
References
-
- Release Notice Canadian Cancer Statistics: A 2020 Special Report on Lung Cancer. [(accessed on 2 May 2024)]. Available online: https://www.canada.ca/en/public-health/services/reports-publications/hea....
-
- Goldstraw P., Chansky K., Crowley J., Rami-Porta R., Asamura H., Eberhardt W.E., Nicholson A.G., Groome P., Mitchell A., Bolejack V., et al. The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

