Role of Sclerostin in Cardiovascular System
- PMID: 40429697
- PMCID: PMC12111627
- DOI: 10.3390/ijms26104552
Role of Sclerostin in Cardiovascular System
Abstract
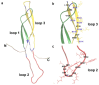
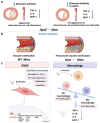
Sclerostin, encoded by the SOST gene, is a novel bone anabolic target for bone diseases. Humanized anti-sclerostin antibody, romosozumab, was approved for treatment of postmenopausal osteoporosis by the US Food and Drug Administration (FDA), but with a black-box warning on cardiovascular risk. The clinical data regarding cardiovascular events from various pre-marketing and post-marketing studies of romosozumab were inconsistent. Overall, the cardiovascular risk of sclerostin inhibition could not be excluded. The restriction of romosozumab in patients with cardiovascular disease history would be necessary. Moreover, genome-wide association study (GWAS) analyses of SOST variants revealed inconsistent results of the association between SOST variations and cardiovascular diseases. Further research incorporating larger sample sizes and functional analyses are necessary. In analyses of serum/tissue sclerostin levels in patients with cardiovascular diseases, the results were controversial but indicated an association between sclerostin and the presence/severity/outcomes of cardiovascular diseases. Nonclinical studies in rodents indicated the inhibitory effect of sclerostin on inflammation, aortic aneurysm, atherosclerosis, and vascular calcification. Sclerostin loop3 participated in the inhibitory effect of sclerostin on bone formation, while the cardiovascular protective effect of sclerostin was independent of sclerostin loop3. Macrophagic sclerostin loop2-apolipoprotein E receptor 2 (ApoER2) interaction participated in the inhibitory effect of sclerostin on inflammation in vitro. Sclerostin in human aortic smooth muscle cells participated in the reduction in calcium deposition. The role of sclerostin in cardiovascular system deserves further investigation.
Keywords: cardiovascular system; clinical data; molecular understanding; nonclinical data; sclerostin.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article. To avoid potential conflict of interest, we would like to request to exclude reviewers who are related to the R&D of humanized therapeutic antibody against sclerostin (romosozumab).
Figures
References
-
- Lewiecki E.M., Blicharski T., Goemaere S., Lippuner K., Meisner P.D., Miller P.D., Miyauchi A., Maddox J., Chen L., Horlait S. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J. Clin. Endocrinol. Metab. 2018;103:3183–3193. doi: 10.1210/jc.2017-02163. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2018YFA0800804/National Key R&D Program of China
- HKBU 12102223, HKBU 12102524, HKBU 12100921/Hong Kong General Research Fund
- CUHK 14108322, CUHK 14109721, CUHK 14103121, CUHK 14103420/Hong Kong General Research Fund
- 82300988/Young Scientists Fund of the National Natural Science Foundation of China
- PDFS2122-2S03/Hong Kong RGC Postdoctoral Fellowship Scheme
LinkOut - more resources
Full Text Sources

