N-N-Substituted Piperazine, Cmp2, Improves Cognitive and Motor Functions in 5xFAD Mice
- PMID: 40429735
- PMCID: PMC12111198
- DOI: 10.3390/ijms26104591
N-N-Substituted Piperazine, Cmp2, Improves Cognitive and Motor Functions in 5xFAD Mice
Abstract
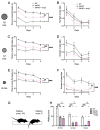
The piperazine derivative N-(2,6-difluorophenyl)-2-(4-phenylpiperazin-1-yl)propanamide (cmp2) has emerged as a potential transient receptor potential cation channel, subfamily C, member 6 (TRPC6) modulator, offering a promising pathway for Alzheimer's disease (AD) therapy. Our recent findings identify cmp2 as a novel compound with synaptoprotective effects in primary hippocampal cultures and effective blood-brain barrier (BBB) penetration. In vivo studies demonstrate that cmp2 (10 mg/kg, intraperitoneally) restores synaptic plasticity deficits in 5xFAD mice. This study further shows cmp2's selectivity towards tetrameric TRPC6 channel in silico. Acute administration of cmp2 is non-toxic, with no indications of chronic toxicity, and Ames testing confirms its lack of mutagenicity. Behavioral assays reveal that cmp2 improves cognitive functions in 5xFAD mice, including increased novel object recognition, better passing of the Morris water maze, and improved fear memory, as well as upregulation of motor function in beam walking tests. These findings suggest that cmp2 holds promise as a candidate for AD treatment.
Keywords: Alzheimer’s disease; TRPC6 channel; behavior; piperazine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

