Early Zinc Supplementation Enhances Epididymal Sperm Glycosylation, Endocrine Activity, and Antioxidant Activity in Rats Exposed to Cadmium
- PMID: 40429736
- PMCID: PMC12111536
- DOI: 10.3390/ijms26104589
Early Zinc Supplementation Enhances Epididymal Sperm Glycosylation, Endocrine Activity, and Antioxidant Activity in Rats Exposed to Cadmium
Abstract
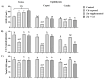
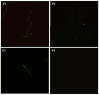
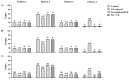
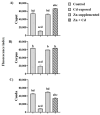
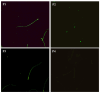
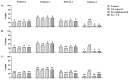
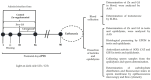
Sperm maturation involves changes in plasma membrane glycosylation for fertilization. Cadmium (Cd) exerts a negative effect by disrupting testicular and epididymal function, altering antioxidant activity. Zinc (Zn) is an essential element known for its antioxidant properties, role in testosterone synthesis, and support of spermatogenesis. However, its effect on sperm membrane glycosylation, as well as endocrine and antioxidant activity, after exposure to Cd has remained unexplored. This study evaluated the impact of Zn on epididymal sperm glycosylation, endocrine activity, and antioxidant activity in Cd-exposed rats. Four groups of male Wistar rats were analyzed: control, Cd-exposed, Zn-supplemented, and Zn + Cd groups. On postnatal day 90, tissues and blood were collected for Zn and Cd quantification, testosterone levels, antioxidant activity, histological analysis, and sperm quality. The results showed that Cd concentration increased significantly, reduced testosterone levels, modified antioxidant activity, and caused structural damage in the epididymis. The Cd-exposed group showed disrupted glycosylation and distribution patterns and reduced sperm quality. The Zn + Cd group showed lower Cd accumulation, preserved testosterone levels, restored antioxidant activity, and preserved glycosylation patterns and sperm quality. This study highlights the protective role of Zn in mitigating Cd-induced reproductive toxicity, probably through the competitive inhibition of Cd uptake and antioxidant support, thereby preserving fertility.
Keywords: cadmium; glycosylation; sperm maturation; spermatogenesis; zinc.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
The protective effects of zinc in lead-induced testicular and epididymal toxicity in Wistar rats.Toxicol Ind Health. 2017 Mar;33(3):265-276. doi: 10.1177/0748233716637543. Epub 2016 Jul 9. Toxicol Ind Health. 2017. PMID: 27102426
-
A Polyherbal Remedy, PurXcel improves Cadmium-induced Male Reproductive Impairment and Testicular Antioxidant Status in Wistar Rats.Niger J Physiol Sci. 2024 Jun 30;39(1):125-135. doi: 10.54548/njps.v39i1.16. Niger J Physiol Sci. 2024. PMID: 40156807
-
Evaluation of the Reversibility of Cadmium-Induced Testicular Toxicity Following Recovery Alone or with Zinc Supplementation.Int J Environ Res Public Health. 2025 Mar 20;22(3):454. doi: 10.3390/ijerph22030454. Int J Environ Res Public Health. 2025. PMID: 40238580 Free PMC article.
-
Combination treatment of zinc and selenium intervention ameliorated BPA-exposed germ cell damage in SD rats: elucidation of molecular mechanisms.Naunyn Schmiedebergs Arch Pharmacol. 2024 Sep;397(9):6685-6704. doi: 10.1007/s00210-024-03044-4. Epub 2024 Mar 18. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38498059
-
Zinc and Its Impact on the Function of the Testicle and Epididymis.Int J Mol Sci. 2024 Aug 19;25(16):8991. doi: 10.3390/ijms25168991. Int J Mol Sci. 2024. PMID: 39201677 Free PMC article. Review.
References
-
- Vickram S., Rohini K., Srinivasan S., Nancy Veenakumari D., Archana K., Anbarasu K., Jeyanthi P., Thanigaivel S., Gulothungan G., Rajendiran N., et al. Role of Zinc (Zn) in Human Reproduction: A Journey from Initial Spermatogenesis to Childbirth. Int. J. Mol. Sci. 2021;22:2188. doi: 10.3390/ijms22042188. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

