Fingolimod Prevents Neuroinflammation but Has a Limited Effect on the Development of Ataxia in a Mouse Model for SCA1
- PMID: 40429839
- PMCID: PMC12111356
- DOI: 10.3390/ijms26104698
Fingolimod Prevents Neuroinflammation but Has a Limited Effect on the Development of Ataxia in a Mouse Model for SCA1
Abstract
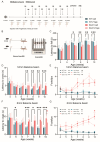
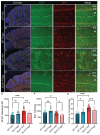
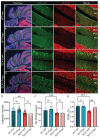
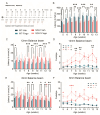
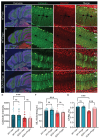
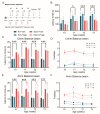
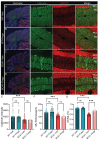
Spinocerebellar ataxia type 1 (SCA1) is a neurodegenerative disorder that predominantly affects the Purkinje cells (PCs) of the cerebellum, leading to cerebellar degeneration, motor dysfunction, and cognitive impairment. Sphingosine-1-phosphate (S1P) signaling, known to modulate neuroinflammation, has been identified as a potential therapeutic target in SCA1. To investigate the therapeutic efficacy of the S1P modulator fingolimod, we treated a mouse model for SCA1, ATXN1[82Q]/+ mice during three different periods with fingolimod and assessed the effects. Potential therapeutic effects were monitored by tracking locomotion during the treatment period and examining PC morphology, connectivity, and markers for neuroinflammation post-mortem. Fingolimod treatment reduced astrocyte and microglial activation during all three treatment periods. We found no effect on calbindin levels or the thickness of the molecular layer, but fingolimod did improve the extent of the synaptic input of climbing fibers to PCs. While fingolimod improved important aspects of cellular pathology, we could only detect signs of improvement in the locomotion phenotype when treatment started at a later stage of the disease. In conclusion, fingolimod is able to mitigate neuroinflammation, preserve aspects of PC function in SCA1, and remediate part of the ataxia phenotype when treatment is appropriately timed. Although behavioral benefits were limited, targeting S1P pathways represents a potential therapeutic strategy for SCA1. Further studies are needed to optimize treatment regimens and assess long-term outcomes.
Keywords: fingolimod; microglia; neuroinflammation; sphingosine-1-phosphate pathway; spinocerebellar ataxia type 1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Horber V., Andersen G.L., Arnaud C., De La Cruz J., Dakovic I., Greitane A., Hensey O., Himmelmann K., Hollody K., Horridge K., et al. Prevalence, Clinical Features, Neuroimaging, and Genetic Findings in Children with Ataxic Cerebral Palsy in Europe. Neurology. 2023;101:e2509–e2521. doi: 10.1212/WNL.0000000000207851. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

