Chronic Chemogenetic Activation of Astrocytes in the Murine Mesopontine Region Leads to Disturbances in Circadian Activity and Movement
- PMID: 40429935
- PMCID: PMC12111845
- DOI: 10.3390/ijms26104793
Chronic Chemogenetic Activation of Astrocytes in the Murine Mesopontine Region Leads to Disturbances in Circadian Activity and Movement
Abstract
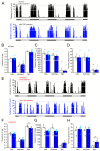
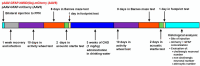
We have previously shown that neuromodulatory actions on astrocytes can elicit metabotropic glutamate- and N-methyl-D-aspartate receptor-dependent tonic changes in excitability in the mesopontine region. Although in vitro experiments explored robust effects, the in vivo significance of our findings remained unknown. In this project, chronic chemogenetic activation of mesopontine astrocytes and its actions on movement, circadian activity, acoustic startle and spatial memory were tested. The control group of young adult male mice where mesopontine astrocytes expressed only the mCherry fluorescent tag was compared to the group expressing the hM3D(Gq) chemogenetic actuator. Chronic chemogenetic astrocyte activation reduced the amplitude of the acoustic startle reflex and increased the locomotion speed in the resting period. Gait alterations were also demonstrated but no change in the spatial memory was explored. As a potential background of these findings, chronic astrocytic activation decreased the cholinergic neuronal number to 54% and reduced the non-cholinergic neuronal number to 76% of the control. In conclusion, chronic astrocytic activation and the consequential decrease in the neuronal number led to disturbances in movement and circadian activity resembling brainstem-related symptoms of progressive supranuclear palsy, raising the possibility that astrocytic overactivation is involved in the pathogenesis of this disease.
Keywords: acoustic startle; activity cycles; astrocyte; chemogenetics; gait; mesopontine region; pedunculopontine nucleus; spatial memory.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures




Similar articles
-
KCNQ4 potassium channel subunit deletion leads to exaggerated acoustic startle reflex in mice.Neuroreport. 2023 Mar 1;34(4):232-237. doi: 10.1097/WNR.0000000000001883. Epub 2023 Feb 6. Neuroreport. 2023. PMID: 36789839 Free PMC article.
-
Quantification of cholinergic and select non-cholinergic mesopontine neuronal populations in the human brain.Neuroscience. 1999 Mar;89(3):759-70. doi: 10.1016/s0306-4522(98)00380-7. Neuroscience. 1999. PMID: 10199611
-
Hippocampal astrocytes induce sex-dimorphic effects on memory.Cell Rep. 2024 Jun 25;43(6):114278. doi: 10.1016/j.celrep.2024.114278. Epub 2024 May 24. Cell Rep. 2024. PMID: 38795347 Free PMC article.
-
Brainstem control of locomotion and muscle tone with special reference to the role of the mesopontine tegmentum and medullary reticulospinal systems.J Neural Transm (Vienna). 2016 Jul;123(7):695-729. doi: 10.1007/s00702-015-1475-4. Epub 2015 Oct 26. J Neural Transm (Vienna). 2016. PMID: 26497023 Free PMC article. Review.
-
The role of astrocytes in generating circadian rhythmicity in health and disease.J Neurochem. 2021 Apr;157(1):42-52. doi: 10.1111/jnc.15312. Epub 2021 Mar 3. J Neurochem. 2021. PMID: 33539604 Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

