On the Potential Role of Phytate Against Neurodegeneration: It Protects Against Fe3+-Catalyzed Degradation of Dopamine and Ascorbate and Against Fe3+-Induced Protein Aggregation
- PMID: 40429940
- PMCID: PMC12112605
- DOI: 10.3390/ijms26104799
On the Potential Role of Phytate Against Neurodegeneration: It Protects Against Fe3+-Catalyzed Degradation of Dopamine and Ascorbate and Against Fe3+-Induced Protein Aggregation
Abstract
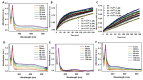
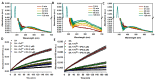
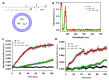
Myo-inositol-1,2,3,4,5,6-hexakisphosphate (IP6) is commonly found in plant-derived foods and has important pharmacological properties against many pathologies. One of them appears to be neurodegeneration, which is notably stimulated by dysregulated metal metabolism. Consequently, we explore the role of IP6 in mitigating neurodegenerative events catalyzed by dysregulated free iron. More precisely, we performed spectrophotometric measurements in aqueous solutions to investigate the ability of IP6 to chelate Fe3+ and inhibit its role in catalyzing the oxidative degradation of dopamine and ascorbic acid, two key molecules in neuronal redox systems. Our results demonstrate that IP6 effectively prevents the formation of harmful intermediates, such as neuromelanin and reactive oxygen species, which are linked to neuronal damage. Additionally, we assessed the effect of IP6 on Fe3+-induced protein aggregation, focusing on α-synuclein, which is closely associated with Parkinson's disease. Our data reveal that IP6 accelerates the conversion of toxic α-synuclein oligomers into less harmful amyloid fibrils, thereby reducing their neurotoxic potential. Our findings highlight the dual function of IP6 as a potent Fe3+ chelator and modulator of protein aggregation pathways, reinforcing its potential as a neuroprotective agent. Consequently, IP6 offers promising therapeutic potential for mitigating the progression of neurodegenerative disorders such as Parkinson's and Alzheimer's diseases.
Keywords: ascorbic acid; dopamine; phytic acid; α-synuclein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Neuroprotective effect of the natural iron chelator, phytic acid in a cell culture model of Parkinson's disease.Toxicology. 2008 Mar 12;245(1-2):101-8. doi: 10.1016/j.tox.2007.12.017. Epub 2007 Dec 27. Toxicology. 2008. PMID: 18255213
-
Neuroprotection of inositol hexaphosphate and changes of mitochondrion mediated apoptotic pathway and α-synuclein aggregation in 6-OHDA induced parkinson's disease cell model.Brain Res. 2016 Feb 15;1633:87-95. doi: 10.1016/j.brainres.2015.12.035. Epub 2015 Dec 29. Brain Res. 2016. PMID: 26740400
-
Complex of EGCG with Cu(II) Suppresses Amyloid Aggregation and Cu(II)-Induced Cytotoxicity of α-Synuclein.Molecules. 2019 Aug 14;24(16):2940. doi: 10.3390/molecules24162940. Molecules. 2019. PMID: 31416122 Free PMC article.
-
Toxic Feedback Loop Involving Iron, Reactive Oxygen Species, α-Synuclein and Neuromelanin in Parkinson's Disease and Intervention with Turmeric.Mol Neurobiol. 2021 Nov;58(11):5920-5936. doi: 10.1007/s12035-021-02516-5. Epub 2021 Aug 23. Mol Neurobiol. 2021. PMID: 34426907 Review.
-
What have we learnt from CDNA microarray gene expression studies about the role of iron in MPTP induced neurodegeneration and Parkinson's disease?J Neural Transm Suppl. 2003;(65):73-88. doi: 10.1007/978-3-7091-0643-3_5. J Neural Transm Suppl. 2003. PMID: 12946050 Review.
References
-
- Chen Y., Yuan W., Xu Q., Reddy M.B. Neuroprotection of phytic acid in Parkinson’s and Alzheimer’s disease. J. Funct. Foods. 2023;110:105856. doi: 10.1016/j.jff.2023.105856. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

