Recent Advances in Nano-Drug Delivery Strategies for Chalcogen-Based Therapeutic Agents in Cancer Phototherapy
- PMID: 40429960
- PMCID: PMC12112061
- DOI: 10.3390/ijms26104819
Recent Advances in Nano-Drug Delivery Strategies for Chalcogen-Based Therapeutic Agents in Cancer Phototherapy
Abstract
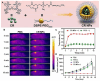
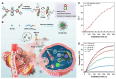
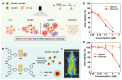
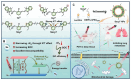
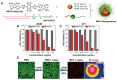
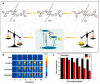
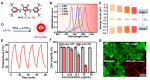
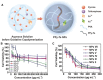
Chalcogen-containing therapeutic agents (TAs), which include sulfur (S), selenium (Se), and tellurium (Te) atoms, have recently emerged as a promising class of photosensitizers (PSs) and photothermal agents (PTAs) for cancer phototherapy. The incorporation of heavier chalcogens into organic chromophores leads to visible-to-near-infrared (VIS-NIR) light absorption, efficient triplet harvesting, and adequate heat and energy transfer efficiency, all of which are paramount for photodynamic therapy (PDT) and photothermal therapy (PTT). However, chalcogen-based PSs/PTAs suffer from photostability, bioavailability, and targeted delivery issues, which minimize their PDT/PTT performances. Nevertheless, significant progress in the rational design of nanoencapsulation strategies has been achieved to overcome the challenges of chalcogen-based TAs for effective phototherapeutic cancer treatment. This review highlights the recent advances (within the last five years) in nano-drug delivery approaches adapted for chalcogen-substituted PSs/PTAs for PDT, PTT, or synergistic PDT/PTT, integrating imaging and treatment. The PSs/PTAs described in this review are classified into three classes: (i) sulfur, (ii) selenium, and (iii) tellurium-containing TAs used in phototherapy applications. This review offers a comprehensive perspective on the design of chalcogen-substituted photosensitizers (PSs) and photothermal agents (PTAs), covering spectroscopic and computational characterization, nanoformulation strategies, and their roles in enhancing reactive oxygen species (ROS) generation and photothermal conversion efficiency for improved in vitro and in vivo performance. We hope this work will encourage further research into nanotechnological strategies designed to enhance the phototherapeutic efficacy of chalcogen-containing therapeutic agents.
Keywords: cancer therapy; chalcogen; nano-drug delivery; photodynamic therapy; photothermal therapy; reactive oxygen species; triplet harvesting.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

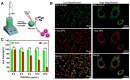
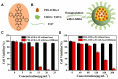
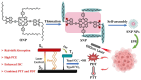
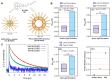


References
-
- Murray S.G., Hartley F.R. Coordination chemistry of thioethers, selenoethers, and telluroethers in transition-metal complexes. Chem. Rev. 1981;81:365–414. doi: 10.1021/cr00044a003. - DOI
-
- Kaur M., Rob A., Caton-Williams J., Huang Z. Biochalcogen Chemistry: The Biological Chemistry of Sulfur, Selenium, and Tellurium. Volume 1152. American Chemical Society; Washington, DC, USA: 2013. Biochemistry of nucleic acids functionalized with sulfur, selenium, and tellurium: Roles of the single-atom substitution; pp. 89–126.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

