Gap Junctional Interaction of Endothelial Progenitor Cells (EPC) with Endothelial Cells Induces Angiogenic Network Formation In Vitro
- PMID: 40429968
- PMCID: PMC12112054
- DOI: 10.3390/ijms26104827
Gap Junctional Interaction of Endothelial Progenitor Cells (EPC) with Endothelial Cells Induces Angiogenic Network Formation In Vitro
Abstract
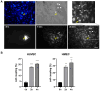
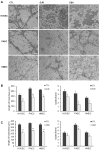
Endothelial progenitor cells (EPC) are considered to support neovascularization and endothelial repair by being incorporated into newly formed or injured vessels and by improving vascularization in a paracrine manner by secreting proangiogenic factors. Here, we studied the role of gap junctional communication between EPC and endothelial cells in long-term co-cultures in vitro. The cultivation of endothelial cells together with mouse embryonic EPC (E 7.5) induced the spontaneous formation of angiogenic networks after 3-6 days consisting of both cell types, but not in the respective monocultures, whereas their respective cultivation on a basement matrix induced the formation of tube-like structures, as expected. The angiogenic network formation could not be mimicked by the incubation of endothelial cells with supernatants of EPC only. We therefore hypothesized that direct interaction and cell-cell communication is required to induce the angiogenic network formation in co-cultures with endothelial cells. Expression analysis demonstrated expression of the gap junctional protein connexin 43 (Cx43) in EPC. Moreover, dye injection studies as well as FACS analysis identified gap junctional communication between endothelial cells and EPC. The inhibition of gap junctions by pharmacological blockers significantly reduced the angiogenic network formation, confirming that gap junctional communication between both cell types is required for this process.
Keywords: angiogenesis; connexin; endothelial cells; endothelial progenitor cells; gap junction.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Ratliff B.B., Ghaly T., Brudnicki P., Yasuda K., Rajdev M., Bank M., Mares J., Hatzopoulos A.K., Goligorsky M.S. Endothelial Progenitors Encapsulated in Bioartificial Niches Are Insulated from Systemic Cytotoxicity and Are Angiogenesis Competent. Am. J. Physiol. Renal Physiol. 2010;299:F178–F186. doi: 10.1152/ajprenal.00102.2010. - DOI - PMC - PubMed
-
- Shin H.S., Thakore A., Tada Y., Pedroza A.J., Ikeda G., Chen I.Y., Chan D., Jaatinen K.J., Yajima S., Pfrender E.M., et al. Angiogenic Stem Cell Delivery Platform to Augment Post-Infarction Neovasculature and Reverse Ventricular Remodeling. Sci. Rep. 2022;12:17605. doi: 10.1038/s41598-022-21510-y. - DOI - PMC - PubMed
-
- Kupatt C., Horstkotte J., Vlastos G.A., Pfosser A., Lebherz C., Semisch M., Thalgott M., Buttner K., Browarzyk C., Mages J., et al. Embryonic Endothelial Progenitor Cells Expressing a Broad Range of Proangiogenic and Remodeling Factors Enhance Vascularization and Tissue Recovery in Acute and Chronic Ischemia. FASEB J. 2005;19:1576–1578. doi: 10.1096/fj.04-3282fje. - DOI - PubMed
-
- Vajkoczy P., Blum S., Lamparter M., Mailhammer R., Erber R., Engelhardt B., Vestweber D., Hatzopoulos A.K. Multistep Nature of Microvascular Recruitment of Ex Vivo-Expanded Embryonic Endothelial Progenitor Cells during Tumor Angiogenesis. J. Exp. Med. 2003;197:1755–1765. doi: 10.1084/jem.20021659. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

