Increased ROS and Persistent Pro-Inflammatory Responses in a Diabetic Wound Healing Model (db/db): Implications for Delayed Wound Healing
- PMID: 40430024
- PMCID: PMC12112478
- DOI: 10.3390/ijms26104884
Increased ROS and Persistent Pro-Inflammatory Responses in a Diabetic Wound Healing Model (db/db): Implications for Delayed Wound Healing
Abstract
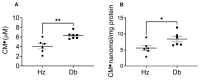
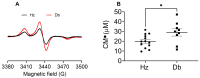
Diabetes and its complications, including impaired wound healing, present a critical clinical challenge and burden for the U.S. healthcare system, with costs of over USD 13 billion annually. Hyperglycemia and chronic inflammation in diabetic wounds increase reactive oxygen species (ROS) production, inducing oxidative stress and perpetuating inflammation, which delays healing. This study investigates inflammation, oxidative stress, and the roles of cellular populations in a diabetic wound healing mouse model (db/db). Given that diabetes leads to persistent inflammation and impaired fibroblast function, we also examined how diabetes influences superoxide production in dermal fibroblasts. Blood, dermal fibroblasts, and wound tissue were collected from 12-week-old female diabetic (Db) and heterozygous (Hz) mice. Electron paramagnetic resonance (EPR) spectroscopy revealed higher superoxide levels in diabetic blood, dermal fibroblasts, and wounds compared to controls. In diabetic wounds, immunohistochemistry and flow cytometry showed increased leukocyte infiltration and reduced macrophage presence, with a higher proportion of pro-inflammatory Ly6Chi macrophages. These results suggest that elevated superoxide production and persistent inflammation contribute to impaired fibroblast function and delayed wound healing in diabetes. By identifying the contributions of ROS and Ly6Chi macrophages to oxidative stress and chronic inflammation, this study offers insights into therapeutic strategies. These findings highlight the importance of addressing systemic oxidative stress alongside localized inflammation to improve wound healing outcomes in diabetic patients and advance diabetic wound care strategies.
Keywords: EPR; chronic inflammation; db/db mouse model; dermal fibroblast; diabetes; reactive oxygen species (ROS); wound healing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Seibold J.R., Uitto J., Dorwart B.B., Prockop D.J. Collagen synthesis and collagenase activity in dermal fibroblasts from patients with diabetes and digital sclerosis. J. Lab. Clin. Med. 1985;105:664–667. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

