NAC1/ACOX2 Axis as a Novel Therapeutic Target for Endometriosis-Related Ovarian Neoplasms
- PMID: 40430078
- PMCID: PMC12112610
- DOI: 10.3390/ijms26104938
NAC1/ACOX2 Axis as a Novel Therapeutic Target for Endometriosis-Related Ovarian Neoplasms
Abstract
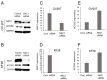
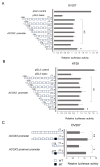
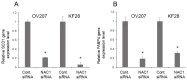
NAC1, a transcription regulator protein associated with cancer, is highly expressed in several tumor types, including ovarian cancer. However, it remains unclear how NAC1 is involved in carcinogenesis. Our previous studies demonstrated that the knockdown of NAC1 in ovarian clear cell carcinoma (OCCC) cell lines induces apoptosis and restores their sensitivity to chemotherapy, suggesting NAC1 as a potential therapeutic target. The present study aimed to identify molecular pathways through which NAC1 is involved in the development of endometriosis-related ovarian neoplasms (ERONs). Immunohistochemistry was performed to clarify the relationship between NAC1 and the potential target protein ACOX2 in surgical specimens of ERONs. Reporter assays were conducted to determine the interaction of NAC1 with the specific cis-element on the ACOX2 promoter. Subsequently, a ChIP assay was performed to investigate the in vivo interaction of NAC1 with the ACOX2 promoter. There was an inverse relationship between NAC1 and ACOX2 expressions in the tumor specimens of ERONs. High NAC1/low ACOX2 expression was found to be a worse prognostic marker for patient survival. Reporter assays demonstrated that NAC1 negatively regulated the ACOX2 promoter via the proximal CATG site. ChIP assays confirmed in vivo binding of NAC1 to the promoter. The present study implicated that NAC1 may contribute to the development of ERONs as a transcriptional repressor by regulating ACOX2 expression via specific binding sites on the promoter, providing a novel insight into the NAC1/ACOX2 axis as a potential therapeutic target of this tumor type.
Keywords: ACOX2; ERON; NAC1; endometriosis; ovarian cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










Similar articles
-
Establishment of a Novel In Vitro and In Vivo Model to Understand Molecular Carcinogenesis of Endometriosis-Related Ovarian Neoplasms.Int J Mol Sci. 2025 Feb 25;26(5):1995. doi: 10.3390/ijms26051995. Int J Mol Sci. 2025. PMID: 40076621 Free PMC article.
-
Fatty acid synthase expression associated with NAC1 is a potential therapeutic target in ovarian clear cell carcinomas.Br J Cancer. 2012 Jul 10;107(2):300-7. doi: 10.1038/bjc.2012.246. Epub 2012 May 31. Br J Cancer. 2012. PMID: 22653145 Free PMC article.
-
Biological role and prognostic significance of NAC1 in ovarian cancer.Gynecol Oncol. 2010 Dec;119(3):469-78. doi: 10.1016/j.ygyno.2010.08.031. Epub 2010 Sep 24. Gynecol Oncol. 2010. PMID: 20869761
-
Pathogenesis and the role of ARID1A mutation in endometriosis-related ovarian neoplasms.Adv Anat Pathol. 2013 Jan;20(1):45-52. doi: 10.1097/PAP.0b013e31827bc24d. Adv Anat Pathol. 2013. PMID: 23232571 Free PMC article. Review.
-
Endometriosis-Associated Ovarian Cancer: From Molecular Pathologies to Clinical Relevance.Int J Mol Sci. 2024 Apr 13;25(8):4306. doi: 10.3390/ijms25084306. Int J Mol Sci. 2024. PMID: 38673891 Free PMC article. Review.
References
-
- Momenimovahed Z., Ghoncheh M., Pakzad R., Hasanpour H., Salehiniya H. Incidence and mortality of uterine cancer and relationship with Human Development Index in the world. Cukurova Med. J. 2017;42:233–240. doi: 10.17826/cutf.322865. - DOI
-
- Maeda D., Chen X., Guan B., Nakagawa S., Yano T., Taketani Y., Fukayama M., Wang T.L., Shih I.-M. Rsf-1 (HBXAP) expression is associated with advanced stage and lymph node metastasis in ovarian clear cell carcinoma. Int. J. Gynecol. Pathol. 2011;30:30–35. doi: 10.1097/PGP.0b013e3181e9a319. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

